Novavax Vaccine Becomes First FDA-Authorized Protein Vaccine for COVID-19
XTalks
JULY 19, 2022
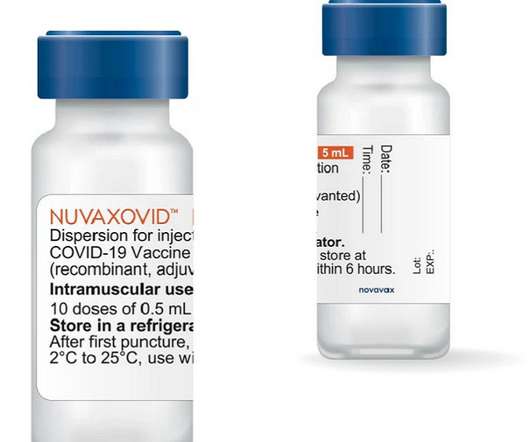
The US Food and Drug Administration (FDA) has given emergency use authorization (EUA) to Novavax’s COVID-19 vaccine, making it the fourth authorized vaccine in the US against COVID-19. The vaccine is branded as Nuvaxovid outside the US with approvals/authorizations in Canada, the EU, UK, Australia and South Korea.






































Let's personalize your content