Risk Assessment for use of Engineered Genetic Materials in Clinical Research
Advarra
MARCH 31, 2023
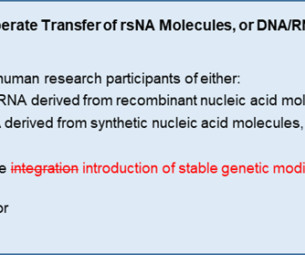
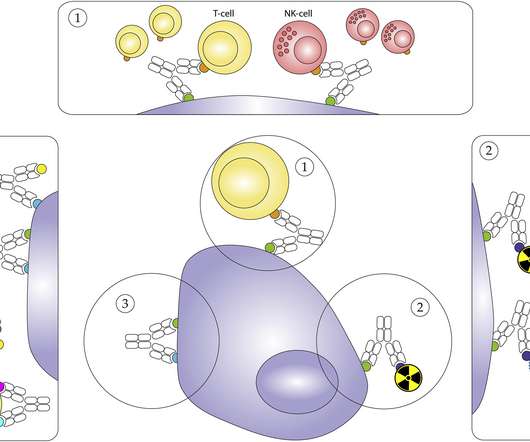
The use of engineered genetic materials in clinical trials is rapidly expanding, with potential applications for genetic vaccines, gene-modified cellular therapies, and gene therapies. A key part of the IBC’s evaluation is assessing the risks posed by the engineered genetic materials.



























Let's personalize your content