Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics
XTalks
FEBRUARY 8, 2024
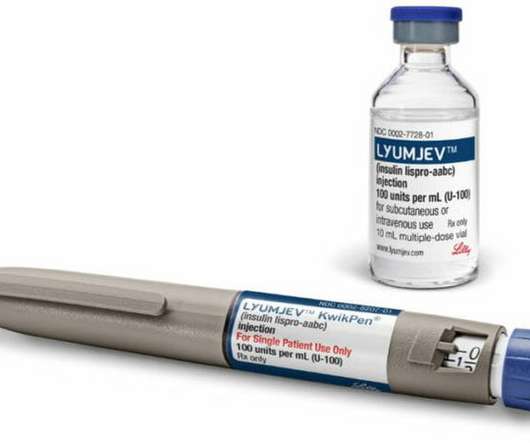
Ozempic (Semaglutide) Ozempic sales in 2022: $8.713 billion Company/Developer: Novo Nordisk Date of first FDA approval: December 5, 2017 Indications Ozempic is FDA-approved for: Type 2 diabetes Price of Ozempic: $1,029 for 1.5 Read on to learn more about the top 15 diabetes drugs in 2023, based on 2022 sales statistics.


















Let's personalize your content