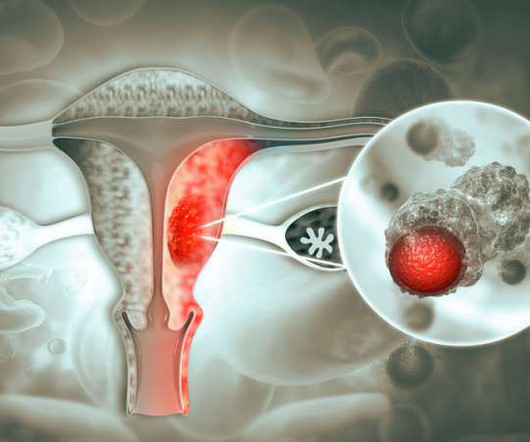
GSK’s Dostarlimab Wins FDA Approval for dMMR Endometrial Cancer
XTalks
APRIL 23, 2021
The therapy is indicated for endometrial cancer that has progressed during, or following, prior treatment with a platinum-based chemotherapy, and in women with dMMR tumors as determined by an FDA-approved test. The immunotherapy received approval as a monotherapy based on GSK’s Biologics License Application (BLA).














































Let's personalize your content