Pfizer takes COVID jab with ‘enhanced’ spike protein into phase 2
pharmaphorum
JULY 27, 2022
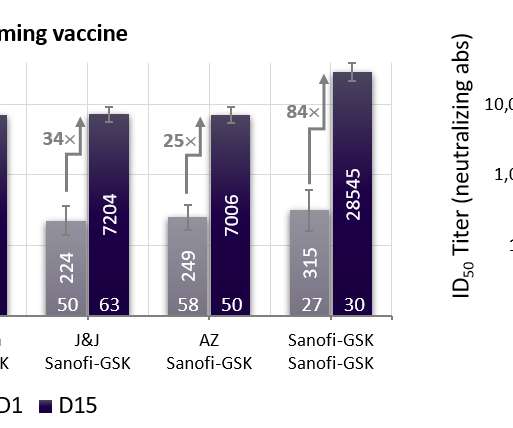
Pfizer and BioNTech have started a mid-stage trial of a new version of the COVID-19 vaccine based on a version of the spike protein that they hope will offer greater and broader protection against SARS-CoV-2 variants. Clinical data show strong neutralising antibody responses against Omicron BA.1,










































Let's personalize your content