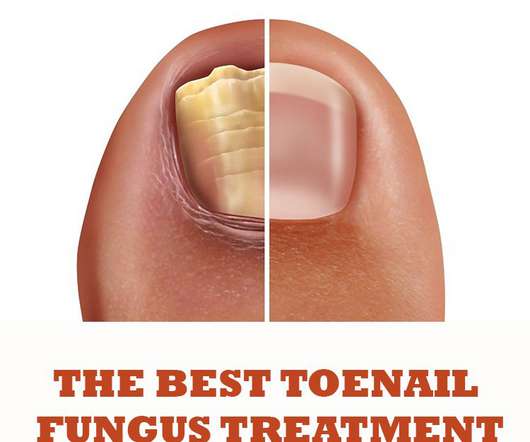
Best Toenail Fungus Treatment Products
Druggist
OCTOBER 21, 2020
Amorolfine 5% nail lacquer (brand name: Curanail 5% w/v Medicated Nail Lacquer) is available as general-sale medication, which can be purchased in most supermarkets and pharmacies. The main products used for toenail fungus treatment are available in most pharmacies, supermarkets and perhaps with the best range of products found online.


























Let's personalize your content