World’s first engineered B cell therapy enters human trials
Drug Discovery World
DECEMBER 19, 2023
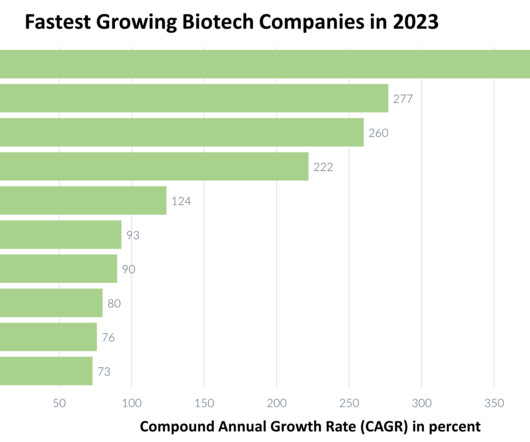
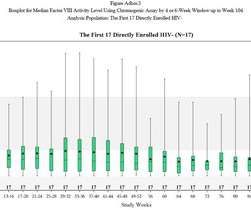
The first patient has been dosed with an engineered B cell investigational therapy in a Phase I trial in Mucopolysaccharidosis type I (MPS I). Developer Immusoft has received FDA Orphan Drug Designation and Rare Pediatric Disease Designation for the therapy, designated ISP-001, in this indication.








































Let's personalize your content