Novo Nordisk submitting once-weekly insulin to FDA following Phase III success
Pharmaceutical Technology
OCTOBER 12, 2022
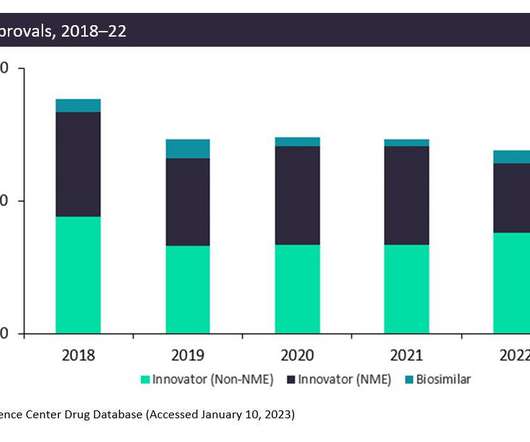
Novo Nordisk has recently completed its six-part ONWARDS Phase III trial, as ONWARDS 5 reached its primary endpoint with Icodec demonstrating non-inferiority in reducing hemoglobin A1C (HbA1c) in patients with type 2 diabetes (T2D) at week 52 in comparison to once-daily basal insulin analogs. Patients had an overall baseline HbA1c of 8.9%







































Let's personalize your content