Meitheal partners with Chinese company for US licencing of insulin biosimilars
Pharmaceutical Technology
SEPTEMBER 21, 2023
The licence grants Meitheal marketing rights for insulin aspart, lispro, and glargine following FDA approval.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
SEPTEMBER 21, 2023
The licence grants Meitheal marketing rights for insulin aspart, lispro, and glargine following FDA approval.

Pharmaceutical Technology
OCTOBER 12, 2022
Novo Nordisk has recently completed its six-part ONWARDS Phase III trial, as ONWARDS 5 reached its primary endpoint with Icodec demonstrating non-inferiority in reducing hemoglobin A1C (HbA1c) in patients with type 2 diabetes (T2D) at week 52 in comparison to once-daily basal insulin analogs. Patients had an overall baseline HbA1c of 8.9%
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JULY 29, 2021
An injectable insulin from Viatris has become the first-ever biosimilar product that can be directly substituted for a marketed biologic, a long-awaited decision that could put pricing pressure on other diabetes drugs.
BioPharma Reporter
MAY 5, 2022
Oramed Pharmaceuticals announced this week that it has enrolled 100% of the patients in the worldâs first Phase 3 study of oral insulin under FDA approved protocols.

pharmaphorum
JULY 29, 2021
Generic drugmaker Mylan has become the first company to secure FDA approval for a biosimilar product that is considered completely interchangeable with the reference product – namely Sanofi’s once-daily insulin Lantus.
pharmaphorum
MAY 16, 2022
It was also compared to a placebo, a GLP-1 receptor agonist (semaglutide) and two long-acting insulin analogues. more than placebo when used in combination with a long-acting insulin. more than insulin degludec and 1.0% more than insulin glargine. more than insulin degludec and 1.0% more than insulin glargine.
The Pharma Data
JULY 29, 2021
Food and Drug Administration approved the first interchangeable biosimilar insulin product, indicated to improve glycemic control in adults and pediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. Biosimilars marketed in the U.S. for the treatment of diabetes.

XTalks
OCTOBER 22, 2021
In July 2021, the agency approved the first interchangeable biosimilar product, Semglee (insulin glargine-yfgn) for the treatment of diabetes. Semglee is both biosimilar to, and interchangeable with, Lantus (insulin glargine). Cyltezo and the Emerging Humira Biosimilars Market.
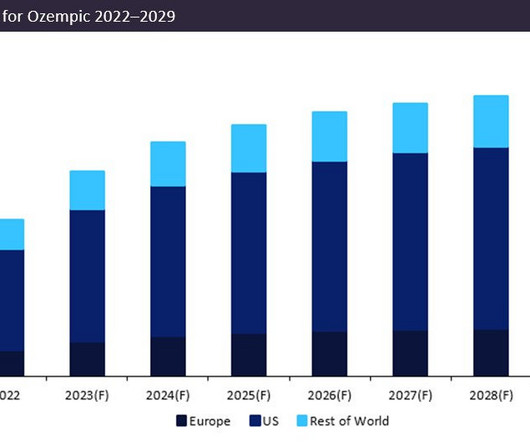
Pharmaceutical Technology
APRIL 28, 2023
Ozempic’s forecast 2023 sales of $12.5bn consolidate its position as the dominant market leader, with projected sales in 2023 54% greater than closest competitor Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8bn. The significant sales growth of Ozempic reinforces its continued dominance in the type 2 diabetes market.

pharmaphorum
JANUARY 28, 2021
It has been five years since the first biosimilar launched in United States market—marking the first steps in expanding access to innovative biologic-based treatments that help patients manage and treat difficult illnesses such as cancer, rheumatoid arthritis, and other life-altering diseases. million patients.

XTalks
FEBRUARY 8, 2024
Understanding the market dynamics of diabetes treatments becomes crucial for professionals across these industries. Ozempic (Semaglutide) Ozempic sales in 2022: $8.713 billion Company/Developer: Novo Nordisk Date of first FDA approval: December 5, 2017 Indications Ozempic is FDA-approved for: Type 2 diabetes Price of Ozempic: $1,029 for 1.5
XTalks
JANUARY 25, 2023
Massachusetts-based TheracosBio has received US Food and Drug Administration (FDA) approval for diabetes med Brenzavvy (bexagliflozin) to help improve glycemic control in adults with type 2 diabetes. It is the first FDA-approved SGLT2 inhibitor for any animal species.

XTalks
NOVEMBER 22, 2022
Provention Bio’s Tzield (teplizumab) has won US Food and Drug Administration (FDA) approval to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients eight years of age and older who have stage 2 type 1 diabetes. In the US, it is estimated that 1.45 million people are currently living with type 1 diabetes.

FDA Law Blog
AUGUST 12, 2021
Koblitz — About two weeks ago, FDA made an exciting announcement (and it remains exciting even if we’re late posting about it): FDA approved the first interchangeable biosimilar. Thus, though Semglee is not new to the market, it is for the first time substitutable for Lantus without intervention of a health care provider.

pharmaphorum
APRIL 1, 2022
market is just beginning to heat up. And already in the first quarter of 2022, the FDA approved a third filgrastim biosimilar, Releuko. With 34 approved biosimilars and dozens more in the pipeline, what does this year have in store? Innovator products working to maintain market share.

Drug Discovery World
JULY 10, 2023
It’s been a fascinating week in drug discovery, as AstraZeneca announced that its Phase III trial of datopotamab deruxtecan did not meet its second primary endpoint and saw an 8% drop in its stock market value as a result.

Drug Discovery World
JULY 10, 2023
It’s been a fascinating week in drug discovery, as AstraZeneca announced that its Phase III trial of datopotamab deruxtecan did not meet its second primary endpoint and saw an 8% drop in its stock market value as a result.

Drug Discovery World
JULY 7, 2023
It’s been a fascinating week in drug discovery, as AstraZeneca announced that its Phase III trial of datopotamab deruxtecan did not meet its second primary endpoint and saw an 8% drop in its stock market value as a result.
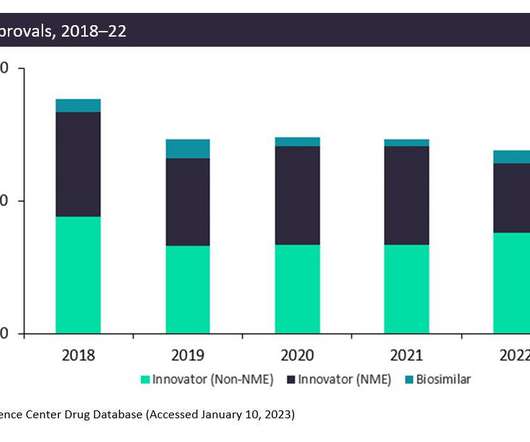
Pharmaceutical Technology
JANUARY 30, 2023
The FDA approved fewer innovative drugs, New Molecular Entities (NMEs), in 2022 than it did in 2021: only 42 drugs compared to 59 drugs. This is due to generally more stringent criteria on approvals in the wake of the Aduhelm scandal. However, non-NME and biosimilar approvals increased in 2022.

XTalks
FEBRUARY 21, 2024
Recently, Eli Lilly revealed promising results from a mid-stage trial, indicating that its popular drug, tirzepatide (marketed as Zepbound and Mounjaro for weight loss and diabetes, respectively), may be an effective treatment for the fatty liver disease metabolic dysfunction-associated steatohepatitis (MASH).

Pharmaceutical Technology
DECEMBER 22, 2022
Financing recognises companies and institutions that have raised significant capital during the research period whether it be through corporate finance, the capital markets or fund raising. The category includes any projects that demonstrate an innovative approach to the market.
pharmaphorum
NOVEMBER 18, 2022
At its second attempt, Provention Bio has secured FDA approval for teplizumab, as a treatment to delay late-stage type 1 diabetes (T1D) in at-risk individuals – becoming the first disease-modifying therapy for these patients. It is now in line for a $35 million equity investment by Sanofi following FDA approval.
STAT News
NOVEMBER 18, 2022
The first therapy that delays the onset of type 1 diabetes received approval from the U.S. The monoclonal antibody teplizumab, which will be marketed under the brand name Tzield and is from ProventionBio and Sanofi, is given through intravenous infusion. Food and Drug Administration , CNN tells us. As of 2019, about 1.9

XTalks
NOVEMBER 3, 2022
Demand for Lilly’s GIP/GLP-1 receptor agonist Mounjaro is also rising because of high patient demand since the drug’s May 13 FDA approval and expanding insurance coverage. In its first quarter on the market since its approval, sales totalled $97 million between July and September in the US.

pharmaphorum
NOVEMBER 21, 2022
The company argues that the cost is justified as Tzield (teplizumab) is the first drug that can delay the onset of type 1 diabetes, fending off the time when they become highly reliant on insulins and at risk of the serious complications that can accompany advanced T1D. It also has an option on global marketing rights to the drug.
The Pharma Data
AUGUST 17, 2021
approval of pump use for Lilly’s novel insulin is latest development designed to help people with diabetes manage blood sugar levels. Lyumjev, a novel formulation of insulin lispro developed to speed the absorption of insulin into the bloodstream and reduce A1C levels, was approved by the FDA in June 2020.
STAT News
DECEMBER 5, 2022
Food and Drug Administration is taking a harder line on its program that fast-tracks drug approvals based on preliminary evidence, spurring GSK, Roche, and others to remake plans for their drugs or pull them from the market , The Wall Street Journal notes. Food and Drug Administration.

pharmaphorum
FEBRUARY 18, 2021
Kellogg’s Corn Flakes had “generic” competition within a month of its launch more than 100 years ago, and yet it’s still the dominant brand in its market. 6 The stakes were extremely high, with annual costs of insulin reaching $736 per patient in 2013, up threefold since 2002. 3) Understand stakeholder drivers in each market.
XTalks
OCTOBER 11, 2023
Rybelsus is Novo’s third semaglutide product on the market, a tablet form of the drug used for the treatment of type 2 diabetes. They mimic the action of GLP-1, a hormone that helps regulate blood sugar levels by enhancing insulin secretion.
pharmaphorum
FEBRUARY 21, 2022
Since the FDA approval, Bayer has also reported the results of a second phase 3 trial – FIGARO-DKD – that concentrated primarily on cardiovascular endpoints and also includes patients with earlier-stage kidney disease.

The Pharma Data
DECEMBER 28, 2020
Amphastar’s newly approved synthetic peptide product was determined by the FDA to be bioequivalent and therapeutically equivalent to Eli Lilly’s Glucagon Emergency Kit for Low Blood Sugar, which has a recombinant DNA (rDNA)-origin. This market information is based on IQVIA data for the 12 months ended September 30, 2020.

Roots Analysis
FEBRUARY 22, 2022
One of the most widely recognized autoinjector in the market is EpiPen® (Mylan), which is a prefilled epinephrine autoinjector. The generic version of EpiPen received the FDA approval in 2018. The market landscape is predominantly characterized by the presence of several small, mid-sized, and large players. Web: [link].

Delveinsight
DECEMBER 7, 2020
Banyan Biomarkers is one company to be able to market a blood-based diagnostic test in the US market, as it utilizes tech to aid the detection of traumatic brain injuries and concussions. In February 2018, the San Diego-based company was granted a de novo request from FDA for the Banyan Brain Trauma Indicator.
pharmaphorum
MARCH 3, 2022
Non-profit drugmaker Civica Rx has said it will launch biosimilars of three big-selling insulin products in the US by 2024 to help diabetic patients struggling with the cost of the drugs. Cheaper options are meanwhile becoming available.

Roots Analysis
JANUARY 14, 2024
Given the ongoing scientific advancements and the rise of FDA-approved biologics, the pharmaceutical industry seems to be approaching the era of biologics. In this competitive global market, outsourcing has evolved into a viable and profitable business model.

XTalks
NOVEMBER 9, 2022
Glucagon-like peptide-1 (GLP-1) receptor agonists are a newer class of diabetes drugs that have the potential to double as weight loss drugs, widening their lucrative market potentials. Novo’s obesity version of semaglutide (at a higher dose) is marketed as Wegovy and was approved in 2021. billion in 2021 to $61.6

pharmaphorum
MARCH 23, 2022
The US Senate will vote in the coming weeks on whether to introduce a $35 cap on the monthly cost of insulin to patients that was endorsed by President Joe Biden in his 2022 State of the Union address. Assuming the bill is later passed by the Senate and signed into law by President Biden, the insulin cap would take effect beginning in 2023.

Camargo
OCTOBER 14, 2020
In this situation, the timing and content of amendments following tentative approval can be complicated; Camargo can help sponsors navigate the process. Approval of the Month: FDA Approves First Closed-Loop Monitoring and Drug Delivery Device.

XTalks
FEBRUARY 2, 2023
Amgen’s Amjevita, the first biosimilar version of AbbVie’s blockbuster arthritis injection Humira (adalimumab), has finally hit the market this week after fetching US Food and Drug Administration (FDA) approval back in 2016. Alvotech’s biosimilar is pending FDA approval. Amjevita Launch: Cost Savings, But for Whom?

XTalks
APRIL 29, 2022
These bind to their respective receptors to increase glucose metabolism by increasing insulin secretion. The promising trial results could pose a threat to Danish drugmaker Novo Nordisk’s GLP-1 agonist drug Wegovy (semaglutide), which has dominated the obesity drug market since its launch in 2021. billion in 2020 to $5.42 percent.

XTalks
AUGUST 2, 2023
Join us as we present an in-depth analysis of each company’s revenue, net income, R&D investments, core therapeutic areas, market presence and strategic collaborations. is a global pharmaceutical company, working across both developed and emerging markets. The FDA approved the drug over a decade ago in September 2009.

XTalks
JUNE 12, 2023
Other approvals included the Intellis rechargeable neurostimulator for treating chronic pain associated with diabetic peripheral neuropathy (DPN). Medtronic’s Hugo Robotic-Assisted Surgery system , released in a limited market in June 2021, has been receiving positive reviews for its performance in various surgeries.

XTalks
SEPTEMBER 17, 2020
Acromegaly Surrogate Endpoint: Serum growth hormone and serum insulin-like growth factor 1 (IGF-1) are acceptable surrogate endpoints for acromegaly clinical trials involving somatostatin analogs such as octreotide, lanreotide and pasireotide. Nizoral is prescribed off-label, while the others are FDA approved.

Drug Discovery World
JULY 13, 2023
The FDA says that it permits some unapproved drugs to be marketed if they are relied on by healthcare professionals to treat serious medical conditions when there is no FDA-approved drug to treat the condition or there is insufficient supply of FDA-approved drugs.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content