Novo, continuing deal streak, buys another obesity drug startup
Bio Pharma Dive
AUGUST 30, 2023
The acquisition of Embark Biotech, which Novo helped found, gives the company another prospect to add to its arsenal of weight-loss medicines.

Bio Pharma Dive
AUGUST 30, 2023
The acquisition of Embark Biotech, which Novo helped found, gives the company another prospect to add to its arsenal of weight-loss medicines.
Pharmaceutical Technology
AUGUST 30, 2023
Biostax and Immgenuity have entered into an agreement to expedite the development of new immunotherapeutics to treat HIV and other diseases.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 30, 2023
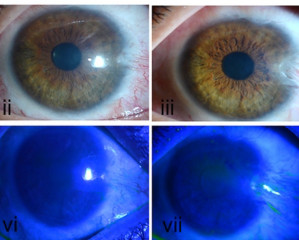
Four patients with severe chemical burns to one eye have shown early positive results in a phase 1 clinical trial of a therapy based on their own stem cells. Even without further treatment, two of the patients reported significant improvements in their vision after a year of follow-up, according to a team of US researchers.

Pharmaceutical Technology
AUGUST 30, 2023
The US FDA has granted priority review for Zealand Pharma’s dasiglucagon for hypoglycemia in the infants with congenital hyperinsulinism.
Bio Pharma Dive
AUGUST 30, 2023
I2o Therapeutics, now led by former Intarcia head Kurt Graves, licensed the diabetes treatment Intarcia has spent more than a decade developing.
Pharmaceutical Technology
AUGUST 30, 2023
The EC has granted approval for Merck’s Keytruda regimen for gastric or gastroesophageal junction (GEJ) adenocarcinoma.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
AUGUST 30, 2023
Shares in the company tanked 80% following the FDA issued a CRL for its bevacizumab formulation.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 30, 2023
The push for Ayurveda, herbal remedies, and nutraceuticals to bridge nutritional gaps reflects a growing interest in holistic health and natural approaches to wellness. In India, healthcare sector has evolved considerably post Covid-19 and there is an increased emphasis on nutraceuticals which have emerged as the way forward.

Pharmaceutical Technology
AUGUST 30, 2023
The subcutaneous Tecentriq has been approved in the UK for all indications that its intravenous counterpart has received approval.
Bio Pharma Dive
AUGUST 30, 2023
The failure marks another Phase 3 miss for the drug, pamrevlumab, and follows a restructuring and CEO switch for the struggling biotech.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Rethinking Clinical Trials
AUGUST 30, 2023
We are excited to welcome I CAN DO Surgical ACP (Improving Completion, Accuracy, and Dissemination of Surgical Advanced Care Planning) to our portfolio of innovative Demonstration Projects. This project is supported within the NIH Pragmatic Trials Collaboratory by a grant from the National Institute on Aging (NIA). Despite advance care planning (ACP) being incorporated into national quality metrics and society guidelines for surgical care for older adults, effective integration of ACP into the p

Bio Pharma Dive
AUGUST 30, 2023
The list of blockbuster cardiovascular, diabetes and cancer drugs gives the industry a window into how regulators are approaching price negotiations.
Pharmaceutical Technology
AUGUST 30, 2023
Alvotech and Bioventure received the Egyptian Drug Authority's approval to produce and supply AVT02 (adalimumab), a biosimilar for Humira.

Fierce Pharma
AUGUST 30, 2023
After 16 years on the market, Takeda's blockbuster attention-deficit/hyperactivity disorder (ADHD) med Vyvanse has finally reached its patent cliff. | After 16 years on the market, Takeda's blockbuster ADHD med Vyvanse has finally reached its patent cliff. And the FDA's approval of several generics comes amid a U.S. shortage of the drug.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
AUGUST 30, 2023
The Phase III trial for FibroGen’s antibody candidate failed to meet its primary endpoint in children with Duchenne muscular dystrophy.

Rethinking Clinical Trials
AUGUST 30, 2023
Speaker Gabriela Schmajuk, MD, MS Professor of Medicine UCSF and the San Francisco VA Slides Keywords Patient-Reported Outcomes, Rheumatoid Arthritis, EHR Key Points Clinicians rely on patient-related outcomes (PROs) to track disease and function over time in patients with rheumatoid arthritis (RA). These outcomes include disease activity, function status, and pain score.

Pharmaceutical Technology
AUGUST 30, 2023
The $1.5bn deal is the largest the newly formed company have performed yet, but more likely to come according to CEO.

Antidote
AUGUST 30, 2023
At the outset, many people may experience apprehension when considering joining a clinical trial. Opting to participate in a research study is a personal one — but it can also be quite gratifying. Various types of clinical studies play a pivotal role in ensuring the approval of life-saving medications and treatments. For this to happen, we need volunteers to take part.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Pharmaceutical Technology
AUGUST 30, 2023
Codagenix faces a late 2024 data readout following enrollment challenges in its paediatric RSV vaccine Phase I study.

Fierce Pharma
AUGUST 30, 2023
Even without an FDA approval, Roche’s cancer drug Avastin is widely used by doctors to treat certain eye diseases. | Even without an FDA approval, Roche’s cancer drug Avastin is widely used by doctors to treat certain eye diseases. Outlook Therapeutics has been trying to get an official approval for a reformulated version, but instead got a rejection letter.
BioSpace
AUGUST 30, 2023
Following the FDA’s recent rejection of zuranolone in major depressive disorder, Sage Therapeutics has launched a strategic reorganization initiative including a 40% reduction in headcount.

Fierce Pharma
AUGUST 30, 2023
Last month, during a quarterly conference call—as Johnson & Johnson considered its future beyond the separation of its consumer health unit—Chief Financial Officer Joe Wolk described the compan | Last month, during a quarterly conference call—as Johnson & Johnson considered its future beyond separation of its consumer health unit—Chief Financial Officer Joe Wolk described the company’s appetite for M&A as “voracious.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Outsourcing Pharma
AUGUST 30, 2023
With more than 63 biopharma companies attending and 15 event partners sharing solutions, the 7th Annual IPF Summit being held in Boston next month (September 2023) is not to be missed.

BioSpace
AUGUST 30, 2023
Amid a supply shortage of the attention-deficit/hyperactivity disorder and binge-eating disorder medication, the regulator has cleared a slew of generic drugs of Takeda’s Vyvanse pill.

BioPharma Reporter
AUGUST 30, 2023
Barbara Morgan is vice president and portfolio executive at public food company Kerry Group, heading up their global pharma and biopharma business.

Pharmaceutical Commerce
AUGUST 30, 2023
Event’s first session aims to analyze the legislation’s implementation, as FDA enforcement is delayed until November 2024.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
AUGUST 30, 2023
The current workforce lacks the skills to move these biological treatments through the regulatory and production pipeline, a new report finds.

Pharma Times
AUGUST 30, 2023
The therapy involves treating individuals with atherosclerotic cardiovascular disease - News - PharmaTimes

Fierce Pharma
AUGUST 30, 2023
After a tumultuous year of manufacturing hiccups, executive turnover and dwindling revenues, Catalent is handing the reins to activist investor Elliott Management. | After a tumultuous year of manufacturing hiccups, executive turnover and dwindling revenues, Catalent is linking up with Elliott Management. The CDMO giant struck a cooperation agreement with the activist investor group as it announced fourth-quarter revenues and unveiled a sweeping governance shakeup.

BioSpace
AUGUST 30, 2023
As the new CEO of LimmaTech Biologics, Haas’ IP and legal background sets him apart to lead the charge for the team’s bacterial vaccine pipeline.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content