US FDA approves Bausch + Lomb and Novaliq’s DED treatment Miebo
Pharmaceutical Technology
MAY 19, 2023
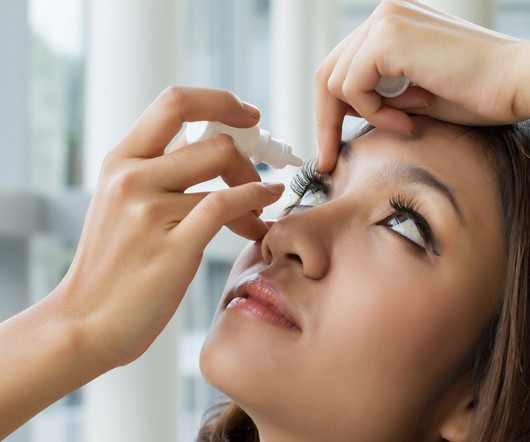
The US Food and Drug Administration (FDA) has granted approval to Bausch + Lomb and Novaliq’s Miebo (perfluorohexyloctane ophthalmic solution) to treat the signs and symptoms of dry eye disease (DED). Formerly known as NOV03, Miebo is a first-in-class eye drop designed for preventing the evaporation of excessive tears and restoring tear balance in evaporative DED patients.





































Let's personalize your content