Point radiopharma drug results disappoint in prostate cancer study
Bio Pharma Dive
DECEMBER 18, 2023
While the study met its goal, the drug’s benefit was less than analysts had predicted, an outcome that could hasten a planned takeover by Eli Lilly.
Bio Pharma Dive
DECEMBER 18, 2023
While the study met its goal, the drug’s benefit was less than analysts had predicted, an outcome that could hasten a planned takeover by Eli Lilly.
Pharmaceutical Technology
DECEMBER 18, 2023
Following US and UK approvals, the EMA’s CHMP has recommended conditional approval for Casgevy, with a decision expected in Q1 2024.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
DECEMBER 18, 2023
The firm is tracking startups that launched during the biotechnology sector’s peak a few years ago but now need fresh funds to move their programs forward, one of its investors said.

Pharmaceutical Technology
DECEMBER 18, 2023
The Novo Nordisk Foundation has invested Dkr1.8bn in the initiative to develop vaccines for various airborne infection diseases.
AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 18, 2023
A healthy smile helps us live long, well and happy lives. But just like our bodies, our teeth succumb to age-related changes. So what happens to teeth as you age? And what can you do to ensure your smile lasts the distance? [link] First, what are teeth made of?

Bio Pharma Dive
DECEMBER 18, 2023
The newly established initiative, which consists of a research center and an accelerator, will focus on developing vaccines for tuberculosis, influenza and group A streptococcus.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Bio Pharma Dive
DECEMBER 18, 2023
Results from a Phase 2 trial fell short of what’s been seen with a similar Eli Lilly medicine, causing the closely watched biotech’s shares to plummet.

Pharmaceutical Technology
DECEMBER 18, 2023
In this issue: Diabetes researchers oppose the regulation of islet transplantation as a biologic in the US, why haemophilia gene therapies are so expensive, how AI will help with trial recruitment.

Bio Pharma Dive
DECEMBER 18, 2023
Activist investor Carl Icahn, who is suing the company over its acquisition of Grail, continued to push for the removal of several board members in a letter to fellow shareholders.

Rethinking Clinical Trials
DECEMBER 18, 2023
Ethics and regulatory onboarding documentation for 2 of the NIH Pragmatic Trials Collaboratory's newest trials is now available. The documents include meeting minutes and supplementary materials summarizing recent discussions of ethics and regulatory issues associated with the ARBOR-Telehealth and I CAN DO Surgical ACP studies. The consultations took place by video conference and included representation from the studies' principal investigators, members of the NIH Collaboratory's Ethics and Regu

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 18, 2023
As antimicrobial resistance (AMR) poses a growing threat to global public health, challenging the effectiveness of antibiotics and other antimicrobial drugs, medical experts suggest whole genome sequencing as a viable option to identify and characterize the bacteria, viruses, fungi or parasites, along with their transmission pattern.

Pharmaceutical Technology
DECEMBER 18, 2023
The US FDA approved Astellas Pharma-Pfizer’s Padcev plus Merck’s (MSD) Keytruda to treat locally advanced or metastatic urothelial cancer.

Rethinking Clinical Trials
DECEMBER 18, 2023
December 10, 2023 : The NIH Pragmatic Trials Collaboratory hosted a Workshop at the 2023 AcademyHealth 16th Annual Conference on the Science of Dissemination and Implementation in Health. This training workshop introduces concepts in the design, conduct, and implementation of embedded pragmatic clinical trials (ePCTs), and provides firsthand ePCT experiences and case studies from the NIH Pragmatic Trials Collaboratory Demonstration Projects.
Pharmaceutical Technology
DECEMBER 18, 2023
Biocytogen Pharmaceuticals and Ona Therapeutics signed an antibody agreement to develop antibody-drug conjugates for solid tumours.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
DECEMBER 18, 2023
To effectively combat rare diseases, it is essential to prioritise the collection and analysis of data. This article explores how pharmaceutical companies are leveraging AI and focusing on orphan drugs to tackle rare diseases.

Pharmaceutical Technology
DECEMBER 18, 2023
The latest $389m pledge follows an initial investment of $300m in 2017 that was raised to fund early-stage life-science startups.
Pharma Times
DECEMBER 18, 2023
MCI patients had 25% lower levels of serotonin compared to healthy patients Researchers from Johns Hopkins Medicine have suggested that serotonin loss in parts of the brain may play a role in cognitive decline in the early stages of Alzheimer’s disease (AD). Published in the Journal of Alzheimer’s Disease , the study was supported by other contributing scientists from the Johns Hopkins University School of Medicine and the Johns Hopkins Bloomberg School of Public Health.
Pharmaceutical Technology
DECEMBER 18, 2023
CymaBay Therapeutics has filed a new drug application (NDA) with the FDA seeking approval for seladelpar for PBC management.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Drug Discovery World
DECEMBER 18, 2023
University College London (UCL) researchers have developed a new gene therapy to cure focal cortical dysplasia, which a new study shows can significantly reduce seizures in mice. Focal cortical dysplasia is caused by areas of the brain that have developed abnormally and is among the most common causes of drug-resistant epilepsy in children. Epilepsy in focal cortical dysplasia is associated with comorbidities, including learning disabilities.

Pharmaceutical Technology
DECEMBER 18, 2023
The EMA CHMP recommended granting approval for Takeda’s Hyqvia for chronic inflammatory demyelinating polyneuropathy.
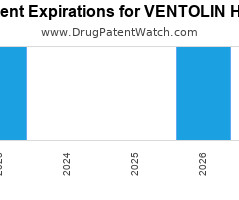
Drug Patent Watch
DECEMBER 18, 2023
Annual Drug Patent Expirations for VENTOLIN+HFA Ventolin Hfa is a drug marketed by Glaxosmithkline and is included in one NDA. It is available from nine suppliers. There are two patents… The post New patent expiration for Glaxosmithkline drug VENTOLIN HFA appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
DECEMBER 18, 2023
Illumina aims to finalise the terms of this divestiture by the end of the second quarter of 2024.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Fierce Pharma
DECEMBER 18, 2023
Following a string of approvals in the United States and beyond, Vertex Pharmaceuticals continues to build out its manufacturing network for the world’s first CRISPR-based gene therapy, Casgevy.
Pharma Times
DECEMBER 18, 2023
MCI patients had 25% lower levels of serotonin compared to healthy patients - News - PharmaTimes

pharmaphorum
DECEMBER 18, 2023
Biogen’s Skyclarys is on course to becoming the first approved medicine for the inherited neurological disease Friedreich’s ataxia (FA) in the EU after it was recommended by the EMA’s human medicines committee.

Pharma Times
DECEMBER 18, 2023
Earlier diagnosis could increase patient survival rates by five years or more - News - PharmaTimes

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Outsourcing Pharma
DECEMBER 18, 2023
Astellasâ drug fezolinetant (Veoza) has been approved by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for the prevention of hot flashes during the menopause.

XTalks
DECEMBER 18, 2023
Merck received its second US Food and Drug Administration (FDA) approval for its cancer pill Welireg (belzutifan). The oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor was first approved in August 2021 for von Hippel-Lindau (VHL), a rare hereditary disease that causes tumor growth in different organs, particularly the kidney. Last week, the drug received its second FDA approval for adults with advanced renal cell carcinoma who have relapsed or have refractory disease following treatment
Outsourcing Pharma
DECEMBER 18, 2023
The oral treatment belzutifan, developed by MSD (known as Merck in the US) and branded as Welireg, has received the green light from the US Food and Drug Administration (FDA) for the treatment of an advanced form of renal cell carcinoma (RCC).
Fierce Pharma
DECEMBER 18, 2023
On a mission to grow in oncology, GSK has more positive data to report in endometrial cancer. | GSK has more positive data to report in endometrial cancer as the company's PD-1/PARP combination turned in a trial win. The combination could represent a threat to a rival therapy at AstraZeneca.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content