5 FDA decisions to watch in the third quarter
Bio Pharma Dive
JUNE 26, 2023
By the end of September, the FDA will hold two anticipated advisory meetings and issue important decisions on drugs for Alzheimer’s, depression and a type of vision loss.

Bio Pharma Dive
JUNE 26, 2023
By the end of September, the FDA will hold two anticipated advisory meetings and issue important decisions on drugs for Alzheimer’s, depression and a type of vision loss.
Pharmaceutical Technology
JUNE 26, 2023
After Intercept’s Ocaliva rejection, multiple companies are taking aim at becoming the first US-approved NASH therapy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 26, 2023
“Cassie” is an anxious adult. She stresses and puts off tasks that should be simple. Seeing others succeed makes her feel inadequate. It’s easier to avoid challenges than risk failing again. She has taken anxiety medication, but it didn’t help much. This hypothetical example illustrates a situation many people have faced.
Bio Pharma Dive
JUNE 26, 2023
Liver enzyme elevations in early- and mid-stage testing have led the drugmaker to discontinue development of lotiglipron and focus on another prospect that’s shown early promise.

Rethinking Clinical Trials
JUNE 26, 2023
Miguel A. Vazquez, MD and George Oliver, MD, PhD presented at the NIH Pragmatic Trials Collaboratory Grand Rounds on June 23, 2023. They presented on the topic of Improving Delivery of Care for Chronic Kidney Disease, Diabetes in Hypertension. Slides and recording from the presentation will be posted at a future date pending publication of a journal article.

Bio Pharma Dive
JUNE 26, 2023
Susan Langer, whose nomination stirred controversy due to her ties to an outgoing director, will now join seven other board members who successfully ran for re-election.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Bio Pharma Dive
JUNE 26, 2023
The biotech is planning a “significant cost reduction” effort in the U.S. following a Phase 3 failure in idiopathic pulmonary fibrosis, the third trial miss it’s reported since May.

Pharmaceutical Technology
JUNE 26, 2023
Managing data privacy and security is essential for the future digitalisation of healthcare. We explore how the sector can adapt to embrace smart medical technologies while preserving patient data confidentiality.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 26, 2023
In order to encourage the sustainable quality in healthcare and patient safety, innovative ideas and implemented practices, the National Accreditation Board for Hospitals and Healthcare Providers (NABH) launched a platform named “NABH Best practices Club” where the NABH accredited/ certified/ applicant hospitals can present and pitch their best practices in their organizations.
Pharmaceutical Technology
JUNE 26, 2023
Lipid nanoparticles are enabling a new generation of engineered cell therapies with a push towards more complex cell engineering and gene editing for allogeneic therapies

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
JUNE 26, 2023
Chromatographic resins are important in the development of antibody therapeutics.
Pharmaceutical Technology
JUNE 26, 2023
Shionogi has signed a definitive agreement for the acquisition of Qpex Biopharma in a deal valued at approximately $140m.

Antidote
JUNE 26, 2023
Every June is designated as Alzheimer’s and Brain Awareness Month , a month dedicated to raising awareness for Alzheimer’s disease and other types of dementias. Alzheimer’s disease is the most common type of dementia , and has impacted humans long before it was officially named in 1910.

Pharmaceutical Technology
JUNE 26, 2023
The company will continue with danuglipron as the competition against Novo Nordisk and Eli Lilly for an oral weight-loss drug intensifies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

XTalks
JUNE 26, 2023
By strategically leveraging content, businesses can drive revenue growth and achieve their financial objectives. Content marketing not only builds trust and credibility but also helps in driving brand awareness which attracts potential customers. Life science companies can position themselves as authoritative sources, create a community of loyal followers and influence purchasing decisions by leveraging content marketing strategies.

Pharmaceutical Technology
JUNE 26, 2023
The FDA has approved Pfizer’s Litfulo for treating young patients with severe alopecia areata, the first available treatment of its kind.

XTalks
JUNE 26, 2023
The processing of fish feces can enable self-sustaining fish and vegetable farms (known as aquaponics) to generate biogas. This biogas can then be reintegrated into the farm’s energy system, contributing to its overall sustainability. Recent groundbreaking research conducted at the University of Gothenburg highlights the remarkable potential of utilizing fish feces for both energy production and plant nourishment.

Pharmaceutical Technology
JUNE 26, 2023
Boehringer and Zealand released Phase II data with the investigational drug survodutide, while Novo Nordisk presented new Ozempic data.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
JUNE 26, 2023
The FDA's review teams were moving against an approval of Sarepta's Duchenne muscular dystrophy gene therapy. | The FDA's review teams were moving against an approval of Sarepta's Duchenne muscular dystrophy gene therapy. But Peter Marks, director of the agency's Center for Biologics Evaluation and Research, disagreed with the staffers' interpretations, coming to a "different conclusion" that led to an eventual approval, a memo shows.

Pharmaceutical Technology
JUNE 26, 2023
CMP Pharma has announced the availability of Atorvaliq for treating high cholesterol and specific heart disease or stroke risk factors.
XTalks
JUNE 26, 2023
Medical device and in vitro diagnostics technologies provider SurModic Inc. has announced that its new drug-coated balloon (DCB) SurVeil has been granted approval by the US Food and Drug Administration (FDA). The balloon is designed for the treatment of patients with peripheral artery disease. The approval comes after the FDA made several requests for additional data, which lengthened the approval process that began in 2021.
Pharmaceutical Technology
JUNE 26, 2023
Gilead Sciences has received positive opinion from the EMA's CHMP for its Trodelvy (sacituzumab govitecan) to treat breast cancer.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioSpace
JUNE 26, 2023
Patients treated in a Phase II study with Lilly’s retatrutide saw up to 24% weight loss at 48 weeks, driven by a triagonist mode of action that can bind and activate the GLP-1, GIP and glucagon receptors.
Pharmaceutical Technology
JUNE 26, 2023
The UK government has announced a lung cancer screening programme to aid in identifying cancer earlier and expedite its diagnosis.

Pharma Times
JUNE 26, 2023
The authorisation would include certain patients with refractory generalised myasthenia gravis - News - PharmaTimes

Pharmaceutical Technology
JUNE 26, 2023
iNova Pharmaceuticals has entered a deal to acquire a consumer healthcare brand portfolio from Mundipharma International.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JUNE 26, 2023
Lundbeck CEO Deborah Dunsire is stepping down on a high note in the neuroscience company’s history. | Lundbeck CEO Deborah Dunsire is retiring on a high note in the neuroscience company’s history. After hitting the company's highest quarterly revenue in the first quarter, the five-year Lundbeck chief executive will pass the baton to UCB's neurology head.

BioSpace
JUNE 26, 2023
Phase II results of MoonLake’s sonelokimab suggest superiority to the competition. Funds raised in the stock offering will support Phase III trials with an anticipated launch in 2027.

Fierce Pharma
JUNE 26, 2023
The potential advantages of a daily-pill version of popular GLP-1 drugs for Type 2 diabetes and obesity are obvious compared to the weekly-injection routine most patients taking these drugs undergo | The potential advantages of a daily-pill version of popular GLP-1 drugs for Type 2 diabetes and obesity are obvious compared to the weekly-injection routine most patients taking these drugs undergo.

Pharma Times
JUNE 26, 2023
Chronic kidney disease affects roughly 700 million patients worldwide - News - PharmaTimes
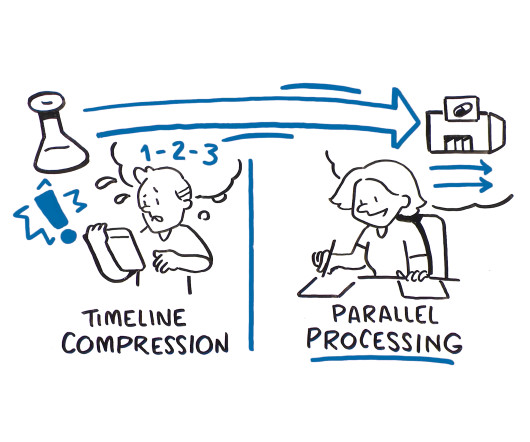
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content