First nonprescription birth control pill approved by FDA
Bio Pharma Dive
JULY 13, 2023
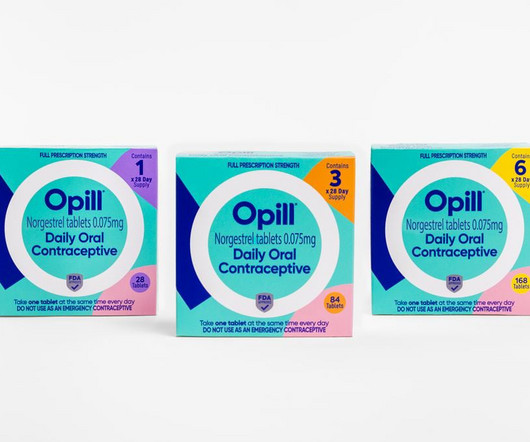
The oral contraceptive’s maker, Perrigo, said the pill will be available in drug and grocery stores early next year, but did not disclose its planned price.
Bio Pharma Dive
JULY 13, 2023
The oral contraceptive’s maker, Perrigo, said the pill will be available in drug and grocery stores early next year, but did not disclose its planned price.

Pharmaceutical Technology
JULY 13, 2023
NVIDIA signed an investment and collaboration agreement with Recursion to create AI models to accelerate drug discovery.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 13, 2023
The world changed for all of us when we were suddenly plunged into a pandemic in 2020. COVID sent us into a series of lockdowns in a bid to control the spread of the virus until a vaccine could be developed.

Rethinking Clinical Trials
JULY 13, 2023
Dr. Adrian Hernandez At the NIH Pragmatic Trials Collaboratory Steering Committee Meeting in May, we interviewed Dr. Adrian Hernandez about how to better promote data sharing. Hernandez is a co–principal investigator of the program’s Coordinating Center and the executive director of the Duke Clinical Research Institute. “We have to change the incentive structure because we still have barriers for sharing data.

Bio Pharma Dive
JULY 13, 2023
Vessey, who previously led research and early drug development at Celgene and Merck, left Bristol Myers earlier this month following changes in the company’s R&D organization.
Pharmaceutical Technology
JULY 13, 2023
Scientists at the ICR, London are set to enter a partnership to merge capabilities for designing precision drugs for cancer leveraging AI.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 13, 2023
The Indian Council of Medical Research (ICMR) has issued list of technologies supported under ICMR-Medical Device and Diagnostics Mission (MDMS) Secretariat to foster indigenous manufacturing of medical devices and diagnostics. This will be implemented through different schemes which will strengthen the healthcare system in line with the ‘Make-in-India’ initiative of the Government of India (GoI).

Pharmaceutical Technology
JULY 13, 2023
Atrial fibrillation drugs' patent expiration is largely responsible for the predicted decline in sales between 2022 and 2032

Rethinking Clinical Trials
JULY 13, 2023
Speaker Ankeet S. Bhatt, MD, MBA, ScM Associate Physician, Kaiser Permanente San Francisco Medical Center Research Scientist, Kaiser Permanente Northern California Division of Research Slides Keywords Heart Failure, Implementation Science, IMPLEMENT-HF Key Points There are 4 drugs that modify 5 pathways, renin-angiotensin inhibition, neprilysin inhibition, SNS inhibition, aldosterone inhibition, and SGLT2 inhibition, that are the mainstays for treatment of heart failure with reduced ejecti
Pharmaceutical Technology
JULY 13, 2023
The company aims to start two trials in H1 2024 based on its current timeline, depending on if it closes its Series B round soon.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

XTalks
JULY 13, 2023
Diagnostic test maker Quanterix has launched LucentAD, a biomarker-based prescription Alzheimer’s disease (AD) test to help in the diagnosis of the disease. The test is intended to help diagnose Alzheimer’s disease with other diagnostic tools. It is not intended as a standalone screening or diagnostic assay, according to Quanterix. LucentAD is designed to evaluate patients experiencing cognitive symptoms that indicate early signs of Alzheimer’s disease.
Pharmaceutical Technology
JULY 13, 2023
Tenpoint Therapeutics has raised Series A funds of $70m for developing engineered cell-based therapies to reverse vision loss.
Pharma Marketing Network
JULY 13, 2023
When it comes to marketing your pharma business on Facebook, you may be wondering whether you should boost a post or create an ad. Both have their own advantages and disadvantages, so it’s important to choose the right option for your needs. Boosting a post is a quick and easy way to get more exposure for your content. When you boost a post, you’re essentially paying to increase its reach and visibility.
Pharmaceutical Technology
JULY 13, 2023
Chinese NMPA has granted approval for AstraZeneca and Daiichi Sankyo’s Enhertu as a monotherapy to treat breast cancer.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
BioPharma Reporter
JULY 13, 2023
Nanjing Iaso Biotherapeutics and Innovent Biologics have received China National Medical Product Administration (NMPA) approval for Fucaso (equecabtagene autoleucel) as a treatment for patients with relapsed or refractory multiple myeloma who have received â3 lines of prior therapies.

Pharmaceutical Technology
JULY 13, 2023
GlobalData has analysed HTA decisions made for branded medicines in 5EU from 2018 to 2022, with a focus on oncology.

Pharma Marketing Network
JULY 13, 2023
The pharmaceutical industry is a highly regulated industry, and as such, marketing strategies must be carefully planned and executed. However, this does not mean that out-of-the-box marketing is not possible or effective. In fact, in some cases, out-of-the-box marketing can be the most effective way to reach target audiences and achieve marketing goals.

Pharmaceutical Technology
JULY 13, 2023
The US FDA has accepted for review BeiGene’s sNDA for Brukinsa plus obinutuzumab to treat follicular lymphoma.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharma Marketing Network
JULY 13, 2023
Your website is your online storefront, so it’s important to choose the best web development company to create it. In the pharmaceutical industry, where trust and credibility are essential, it’s especially important to choose a web development company that understands the specific needs of your business. Here are some factors to consider when choosing a web development company for the pharmaceutical industry: ● Experience in the pharmaceutical industry: Make sure the company you choo
Pharmaceutical Technology
JULY 13, 2023
US FDA granted the IgG1 monoclonal antibody the first qualified infectious disease product (QIDP) designation.

Pharma Times
JULY 13, 2023
Results are from company’s phase 2 head and neck cancer trial with lead NOX inhibitor candidate - News - PharmaTimes

Pharmaceutical Technology
JULY 13, 2023
The non-age-restricted pill is expected to roll out early in 2024, and will ‘lower longstanding barriers’.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Commerce
JULY 13, 2023
Walmart has opened 70 Specialty Pharmacies of the Community locations across the U.S. in June, and is expected to open 80 more in 11 states by the end of the year.

Pharmaceutical Technology
JULY 13, 2023
The investigation combines Ocrevus’ subcutaneous formulation with Halozyme’s drug delivery technology.
Fierce Pharma
JULY 13, 2023
With the multiple sclerosis market growing rapidly—from $18.9 billion in 2020 to a projec | A phase 3 study has shown that a new, subcutaneous version of Roche's Ocrevus has proven to be non-inferior to the current infused treatment as measured by the level of drug in the blood, 12 weeks after administration. An approval for the injected version would allow patients to receive treatment in 10 minutes as opposed to four hours, every six months.

Outsourcing Pharma
JULY 13, 2023
The U.S. company Arthrosi Therapeutics has bagged $75 million in a Series D round to fund the clinical development of a small molecule drug for chronic gout.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JULY 13, 2023
After a full FDA approval last week triggered Medicare coverage of Eisai and Biogen’s Leqembi, doctors are still trying to work out the logistics of testing and reimbursement. | After a full FDA approval last week triggered Medicare coverage of Eisai and Biogen’s Leqembi, doctors are still working out the logistics of testing and reimbursement. But at least two experts remain positive about the drug’s efficacy and safety profile.

Drug Channels
JULY 13, 2023
Today’s guest post comes from Josh Bliss, SVP of Customer Acquisition and Head of Pharma Solutions at SingleCare Josh discusses the challenges that pharmaceutical manufacturers face connecting with consumers who could benefit from patient financial support programs. He describes SingleCare's new Pharma Solutions, which offers manufacturers the opportunity to market their copay assistance programs directly to SingleCare's millions of monthly prescription discount users.
BioSpace
JULY 13, 2023
In a Phase I trial, Caribou’s allogeneic CAR-T cell therapy candidate induced high rates of treatment response in patients with relapsed or refractory B cell non-Hodgkin lymphoma.

Fierce Pharma
JULY 13, 2023
CureVac is adding fuel to its COVID-19 vaccine patent fire by asserting more claims against Pfizer and BioNTech in both its U.S. and German cases. | CureVac is adding more fuel to the fire in its COVID-19 patent cases against Pfizer and fellow German mRNA maker BioNTech, adding a tenth claim in its U.S. dispute and three to its suit in Germany.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content