Gilead’s Sunlenca receives EC approval to treat HIV
Pharmaceutical Technology
AUGUST 23, 2022
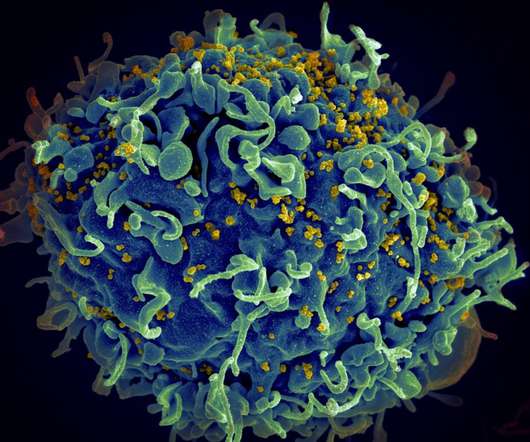
Gilead Sciences has obtained the European Commission (EC) marketing authorisation for Sunlenca (lenacapavir) injection and tablets to treat human immunodeficiency virus (HIV) infection. The twice-yearly treatment is indicated to be administered along with other antiretroviral(s) in adult patients with multi-drug resistant HIV infection who otherwise cannot have a suppressive anti-viral regimen.
































Let's personalize your content