Cultivating quality excellence in the clinical trials landscape
pharmaphorum
NOVEMBER 27, 2023
Cultivating quality excellence in the clinical trials landscape Mike.
pharmaphorum
NOVEMBER 27, 2023
Cultivating quality excellence in the clinical trials landscape Mike.

pharmaphorum
NOVEMBER 28, 2023
Putting patients at the centre of innovation starts with clinical trial access Mike.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
NOVEMBER 29, 2023
Professor Deborah Ashby, interim Dean of the Faculty of Medicine, Imperial College, delivered this yearâs Sally Hollis Memorial Lecture. OSP enjoyed a discussion with both her and Professor Jennifer Visser-Rogers, vice-president for statistical research and consultancy at Phastar, about how new approaches to clinical trials are changing data monitoring committees.

pharmaphorum
NOVEMBER 29, 2023
What the FDA’s groundbreaking guidance really means for psychedelic therapy clinical trials Mike.

pharmaphorum
NOVEMBER 27, 2023
Held at London’s Battersea Arts Centre on 5th October, Sanofi’s ‘What If?’ People’s Poem event coincided with National Poetry Day.

Bio Pharma Dive
NOVEMBER 28, 2023
U.S. tax law changes enacted six years ago slashed large pharma companies' rates and saved them billions. Now, a push for an international floor could disrupt their R&D accounting.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Worldwide Clinical Trials
NOVEMBER 27, 2023
By: Amy Raymond, PMP, PhD, Senior Director, Therapeutic Strategy Lead, Cellular & Genetic Medicines On November 16, the British regulatory body, Medicines and Healthcare products Regulatory Agency (MHRA) approved exagamglogene autotemcel (exa-cel) to treat severe sickle cell disease for patients ages 12 and up, becoming the first gene editing treatment to receive regulatory approval.

Rethinking Clinical Trials
NOVEMBER 29, 2023
In this Friday’s PCT Grand Rounds, Jim Hughes of the University of Washington will continue our special series, Advances in the Design and Analysis of Pragmatic Clinical Trials, with his presentation, “Guidelines for Design and Analysis of Stepped-Wedge Trials.” The session will be held on Friday, December 1, at 1:00 pm eastern. Hughes is a professor emeritus of biostatistics at the University of Washington.
Bio Pharma Dive
NOVEMBER 28, 2023
The agency said the benefit of approved treatments like Gilead’s Yescarta still outweighs any such risk, but the alert could slow drugmaker efforts to develop the treatments for wider use.
Pharmaceutical Technology
NOVEMBER 28, 2023
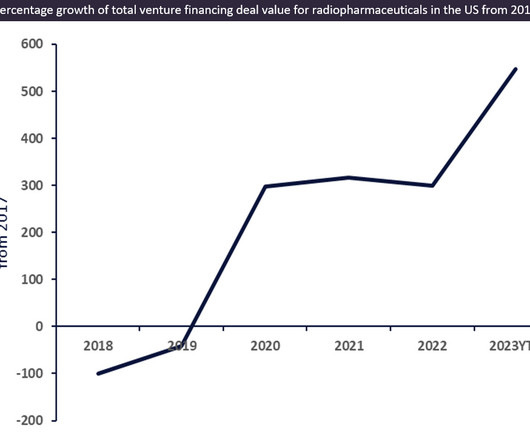
Venture financing for innovative radiopharmaceutical drugs witnessed an approximately 550% increase from $63m in 2017 to $408m in 2023 YTD (year-to-date) total deal value in the US.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Fierce Pharma
NOVEMBER 30, 2023
With layoffs hitting employees on both sides of the Atlantic, Pfizer’s $3.5 billion cost-cutting spree has kicked it into high gear this month. | The company's Groton, Connecticut, research site is the latest to fall victim to job cuts as part of Pfizer's massive $3.5 billion cost-cutting mission, following layoffs across the U.S. and the U.K.

Rethinking Clinical Trials
NOVEMBER 28, 2023
The workshop summary is now available from the NIH Pragmatic Trials Collaboratory’s recent workshop, “Getting the Right Evidence to Decision-Makers Faster.” The 2-day workshop explored the critical cycle of evidence generation by researchers to decision-making by healthcare system leaders to implement the findings of pragmatic clinical trials conducted within healthcare systems.

Bio Pharma Dive
NOVEMBER 29, 2023
Bumpus, a former Johns Hopkins professor, named “creating a new model” for the FDA’s Office of Regulatory Affairs as one of her priorities when she steps into the role.
Pharmaceutical Technology
NOVEMBER 28, 2023
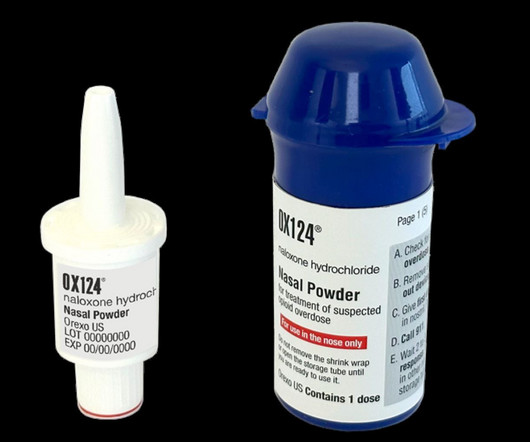
The US Food and Drug Administration (FDA) has accepted to review Orexo’s new drug application (NDA) for opioid overdose medication, OX124.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
NOVEMBER 28, 2023
In a blow to CAR-T therapies, the FDA is investigating a “serious risk” of patients developing new cancers after treatment with these highly efficacious oncology drugs. | In a blow to CAR-T therapies, the FDA is investigating a “serious risk” of patients developing new cancers after treatment with these highly efficacious oncology drugs.

Rethinking Clinical Trials
NOVEMBER 27, 2023
The National Institutes of Health’s Office of Disease Prevention (ODP) issued a new funding opportunity to support implementation studies in treatment and prevention for alcohol, tobacco, and other drugs use and misuse in adult populations that experience health disparities. ODP and participating NIH Institutes and Centers are inviting applications for research projects that test innovative approaches to implementing screening, brief intervention, and referral to treatment or prevention fo

Bio Pharma Dive
NOVEMBER 27, 2023
One year after the U.K. drugmaker withdrew the multiple myeloma drug from the U.S. market because of negative data, new study results might crack open the door to a relaunch.

Pharmaceutical Technology
DECEMBER 1, 2023
Hot on the heels of the designation, Orchard Therapeutics is pressing ahead with a multi-centre trial for the candidate with a start date in December.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Pharma
NOVEMBER 28, 2023
As the pandemic eased earlier this year, officials in Poland became vocal critics of the financial obligations laid down by Pfizer’s large vaccine supply pact with the European Union. | As the pandemic eased earlier this year, officials in Poland became vocal critics of the financial obligations laid down by Pfizer’s large vaccine supply pact with the European Union.

Rethinking Clinical Trials
NOVEMBER 30, 2023
The Health Care Systems Research Network (HCSRN) is accepting abstract submissions and panel presentation submissions for its 2024 Annual Conference until December 11, 2023. This year’s meeting will be held in Milwaukee, Wisconsin, from April 9 to 11, 2024. The HCSRN is a 20-member research network focused on supporting research institutes aligned with healthcare delivery systems.

Bio Pharma Dive
DECEMBER 1, 2023
The EMA's safety committee has more questions for makers of the in-demand therapies as it reviews whether the drugs are linked to the risk of suicidal thoughts.
Pharmaceutical Technology
NOVEMBER 28, 2023
The emergency use listing is based on non-clinical data where the vaccine demonstrated immune responses against variants in SARS-CoV-2.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
NOVEMBER 29, 2023
Seeking to identify ways to improve the discovery and production of medicines, Amgen is expanding its partnership with tech giant Amazon. | Amgen is growing its decade-old collaboration with Amazon Web Services—a widely used cloud platform—to create generative artificial intelligence that the companies aim to use to increase the manufacturing throughput of pharmaceuticals.

BioSpace
NOVEMBER 27, 2023
Building on a previous 2021 collaboration, Bristol Myers Squibb is paying $100 million upfront for the development of five cardiovascular targets utilizing Avidity Biosciences’ antibody oligonucleotide conjugates.

Bio Pharma Dive
NOVEMBER 29, 2023
Drawn-out negotiations led to a lower price than initially expected, but analysts called the agreement a step forward for the biotech company.

Pharmaceutical Technology
NOVEMBER 29, 2023
After months of negotiations, the UK's DHSC and the Association of the British Pharmaceutical Industry emerged with a plan on to replace VPAS.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
NOVEMBER 30, 2023
The sizzling antibody-drug conjugate (ADC) field is at the center of another major life sciences deal. | The sizzling antibody-drug conjugate field is at the center of another major life sciences deal. Hoping to redeem itself following the epic Rova-T failure, AbbVie is shelling out $10.1 billion in cash to acquire ImmunoGen, maker of the ovarian cancer treatment Elahere.

BioPharma Reporter
NOVEMBER 27, 2023
It is five years this month since a ground-breaking change in UK healthcare history happened â with doctors being able to prescribe cannabis-based medicines for the first time.

Bio Pharma Dive
NOVEMBER 29, 2023
The cuts will impact 7% of the cancer drug division’s employees, although about 90 new roles will be created around “areas of growth.

Pharmaceutical Technology
NOVEMBER 28, 2023
A Phase IIb/III trial studying the company’s immunotherapy izokibep, failed to achieve statistical significance for its primary endpoint

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Let's personalize your content