Italfarmaco’s Duvyzat Wins FDA Approval as First Nonsteroidal Treatment for All Genetic Variants of DMD
XTalks
MARCH 28, 2024
Italy-based drugmaker Italfarmaco has won US Food and Drug Administration (FDA) approval for its oral medication Duvyzat (givinostat) for the treatment of Duchenne muscular dystrophy (DMD) in patients six years of age and older. million, which is not unusual for gene therapies, Elevidys has been off to a strong start since its launch.

















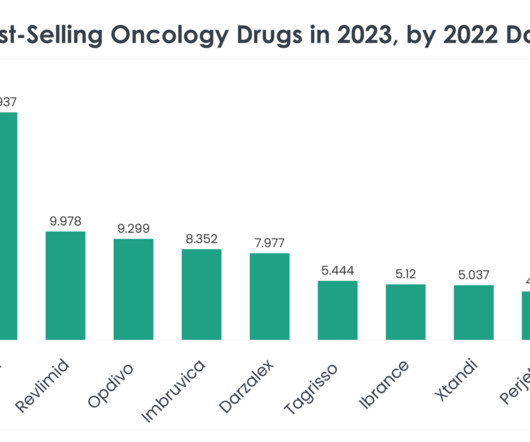





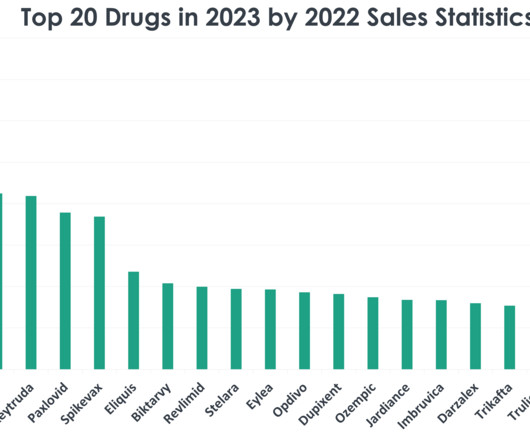















Let's personalize your content