Indian pharma manufacturing: rising investment in Andhra Pradesh
Pharmaceutical Technology
APRIL 4, 2023
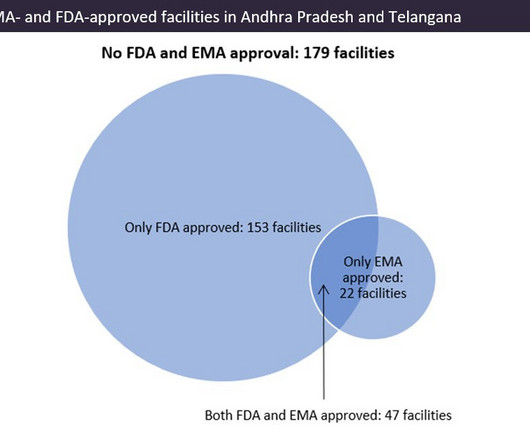
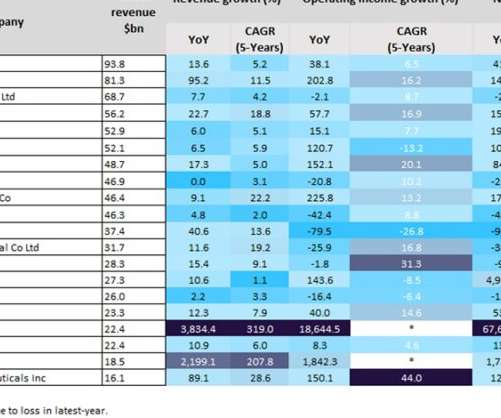
Indian pharma manufacturing continues to be the backbone of drug supplies worldwide, and GlobalData analysis suggests US overreliance on the country for generic drug supply. Pharma manufacturing facilities in Andhra Pradesh and Telangana accounted for 22.5% There are fewer EMA-approved sites in the region.




































Let's personalize your content