New COVID-19 Testing Technology + FDA Approves First Drug for HER2-Low Breast Cancer – Xtalks Life Science Podcast Ep. 72
XTalks
AUGUST 10, 2022
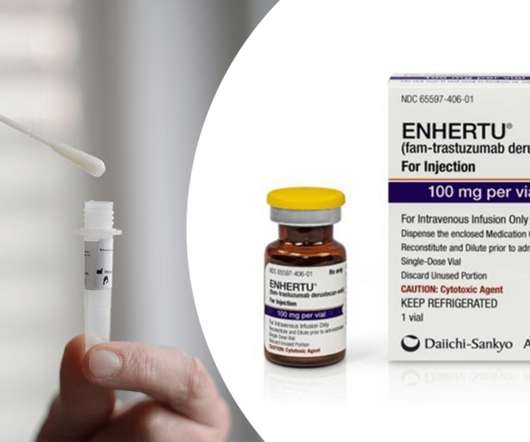
In this episode, Ayesha discussed a new COVID-19 test technology that Innova Medical Group, world leader in at-home COVID-19 tests, has reached a licensing deal for with the University of Birmingham where the technology was developed. AstraZeneca’s Enhertu Gets FDA Approved as First Therapy for HER2-Low Breast Cancer.

































Let's personalize your content