FDA approves new administration routes for Xcopri
Pharmaceutical Technology
APRIL 12, 2024
SK Life Sciences Xcopri (cenobamate) is approved as an oral suspension mixed with water for mouth administration or via a nasogastric tube.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
APRIL 12, 2024
SK Life Sciences Xcopri (cenobamate) is approved as an oral suspension mixed with water for mouth administration or via a nasogastric tube.

XTalks
DECEMBER 7, 2022
In this episode, Ayesha talked about the FDA approval of Ferring Pharmaceuticals’ fecal matter-based therapy Rebyota for the treatment of recurrent C. Read the full articles here: FDA Approves Rebyota as First Fecal Microbiome Therapy for Recurrent C. difficile infections. Difficile Infection.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
FEBRUARY 15, 2023
Bastiaan Driehuys, PhD Founder and Chief Technology Officer Polarean Imaging plc In this week’s Xtalks Life Science podcast episode, Ayesha and the editorial team spoke with Dr. Bastiaan Driehuys, Founder and Chief Technology Officer at Polarean Imaging plc. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

Pharmaceutical Technology
FEBRUARY 2, 2023
Last December, GSK and Wave Life Sciences entered a strategic partnership to drive the discovery and development of oligonucleotide therapies for new genetic targets. The post US FDA approves GSK’s Jesduvroq to treat anaemia of CKD appeared first on Pharmaceutical Technology.

XTalks
SEPTEMBER 14, 2022
In this episode, Ayesha discussed the FDA approval of Sanofi’s enzyme replacement therapy Xenpozyme for the treatment of non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD), a rare genetic lysosomal storage disease, in adults and pediatric patients.

XTalks
NOVEMBER 2, 2022
In this episode, Ayesha discussed the FDA approval of two new immunotherapies, including Janssen/Johnson & Johnson’s bispecific antibody Tecvayli for the treatment of relapsed or refractory multiple myeloma. The drug is the first bispecific T cell antibody to be approved in the US.
XTalks
DECEMBER 14, 2022
In this week’s Xtalks Life Science podcast episode, Ayesha and the team spoke with the co-founders and co-CEOs of Amylyx, Joshua Cohen and Justin Klee, and Amylyx’s General Manager of Canada Chris Aiello, about the recent FDA approval of the company’s ALS drug Relyvrio.

XTalks
AUGUST 3, 2022
In this episode, Ayesha discussed the FDA approval of Azurity Pharmaceutical’s Zonisade (zonisamide oral suspension) as an adjunct therapy for the treatment of seizures in adults and pediatric patients 16 years of age and older with epilepsy. The drug is the first FDA approved oral suspension form of zonisamide.

XTalks
JANUARY 25, 2023
In this week’s Xtalks Life Science podcast episode, Ayesha and the editorial team spoke with Susan Benton, Country Manager at Thea Pharma, an eye care company that focuses on developing innovative eye care treatments to help further the future of ophthalmic treatment. Susan Benton Country Manager Thea Pharma Inc.
XTalks
AUGUST 2, 2023
Eichenfield serves on Verrica Pharmaceuticals’ Board of Directors and spoke to Xtalks about the company’s recent approval of YCANTH (cantharidin) topical solution as the first FDA approved treatment for pediatric and adult patients with molluscum contagiosum, a highly contagious viral skin infection that primarily affects children.

XTalks
AUGUST 23, 2023
BriaCell recently received FDA approval for its Bria-IMT Combination pivotal study design in advanced metastatic breast cancer. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

XTalks
JANUARY 18, 2023
Ayesha also talked about the FDA approval of AstraZeneca’s asthma inhaler Airsupra, the first-in-class drug that contains both a beta agonist and corticosteroid. The inhaler is approved as a rescue medication for adults with asthma to help treat bronchoconstriction and asthma attacks.

Drug Discovery World
MARCH 7, 2023
For International Women’s Day, DDW’s Diana Spencer celebrates some of the women in leadership making an impact on the drug discovery and life sciences sector. The life sciences sector has traditionally outperformed others when it comes to attracting women to its workforce. Dr Loubna Bouarfa, Founder and CEO, OKRA.ai

XTalks
DECEMBER 20, 2023
In 2023, there were significant advancements and notable trends in the life sciences. Xtalks compiled a list of its top life science news and trends of 2023, which provided readers with the latest developments, information and expert insights across life science industries, including pharma, biotech and medical device.

XTalks
AUGUST 31, 2022
In this episode, Ayesha discussed the FDA approval of Axsome Therapeutics’ rapid-acting oral treatment Auvelity for the treatment of major depressive disorder (MDD). The approval makes Auvelity the first and only rapid-acting oral medication for depression, and the first and only oral NMDA receptor antagonist approved for MDD.

XTalks
SEPTEMBER 7, 2022
Ayesha also discussed the FDA approval of Imbruvica (ibrutinib) for pediatric patients one year of age and older with chronic graft-versus-host disease (cGVHD). This is the BTK inhibitor’s first approval for a pediatric indication, and the first approved liquid form of the drug.
XTalks
JUNE 23, 2022
In this episode, Ayesha and the team talked about the FDA approval of Eli Lilly’s JAK inhibitor Olumiant for the treatment of alopecia areata, also commonly just known as alopecia. The drug has become the first approved systemic treatment for the autoimmune disorder that causes patchy hair loss.

XTalks
APRIL 3, 2024
Related: Novartis’ Fabhalta Gets FDA Approval for Rare Complement Blood Disorder The FDA’s green light for Voydeya comes three months after the Japanese Ministry of Health, Labour and Welfare (JHLW) became the first regulator in the world to back the therapy.

XTalks
DECEMBER 8, 2021
The team also talked about a new hepatitis B vaccine from VBI Vaccines that received FDA approval for adults. The vaccine is the first three-antigen hepatitis B vaccine approved in the US. VBI Vaccines Gets FDA Approval for First Three-Antigen Hepatitis B Shot.

XTalks
OCTOBER 19, 2022
The editorial team also learned about the FDA approval of Nevro Corp’s AI-based spinal cord stimulation (SCS) system for the treatment of chronic pain. Nevro’s AI-Based Spinal Cord Stimulation Device for Chronic Pain Receives FDA Approval. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.
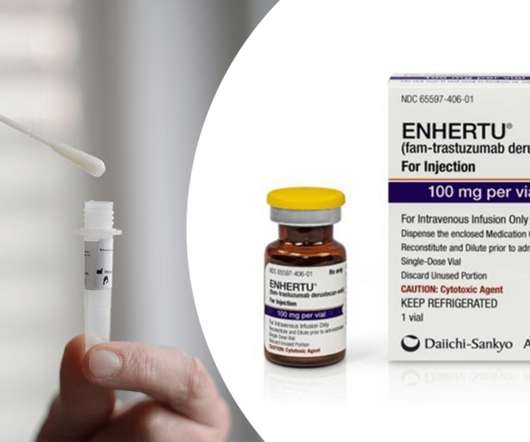
XTalks
AUGUST 10, 2022
Ayesha also talked about the FDA approval of AstraZeneca and Daiichi Sankyo’s antibody-drug conjugate (ADC) Enhertu (trastuzumab-deruxtecan) for the treatment of patients with unresectable or metastatic HER2-low breast cancer. AstraZeneca’s Enhertu Gets FDA Approved as First Therapy for HER2-Low Breast Cancer.

XTalks
MARCH 28, 2024
Italy-based drugmaker Italfarmaco has won US Food and Drug Administration (FDA) approval for its oral medication Duvyzat (givinostat) for the treatment of Duchenne muscular dystrophy (DMD) in patients six years of age and older. Duvyzat received Priority Review, Orphan Drug and Rare Pediatric Disease designations from the FDA.

XTalks
NOVEMBER 23, 2022
In this episode, Ayesha talked about the breakthrough FDA approval of Provention Bio’s Tzield for delaying the onset of type 1 diabetes in adult and pediatric patients. It’s the first approved treatment for slowing the progression of stage 2 type 1 diabetes to stage 3, the stage at which a clinical diagnosis is made.

XTalks
SEPTEMBER 29, 2022
In this episode, Ayesha discussed the FDA approval of bluebird bio’s Skysona for slowing the progression of neurologic dysfunction associated with the rare neurological disorder cerebral adrenoleukodystrophy (CALD) in boys four to 17 years of age with early, active CALD. CDC Expresses Concern Over New COVID-19 Variant BF.7.

XTalks
JANUARY 3, 2024
Wainua is the only FDA-approved drug for the treatment of ATTRv-PN that can be self-administered via an auto-injector. Approval of Wainua represents a meaningful advancement in treatment, one that gives those who are living with transthyretin-mediated amyloid polyneuropathy help managing the disease,” said Michael J.

XTalks
NOVEMBER 28, 2023
This liquid formulation of metronidazole is the sole FDA-approved liquid option, offering a groundbreaking prescribing alternative for patients encountering difficulties in swallowing or facing taste-related obstacles. With a 24-month shelf life and no need for refrigeration, Likmez provides a convenient option for patients.

XTalks
JANUARY 2, 2024
For instance, Vyjuvek , the first FDA-approved gene therapy for DEB, is priced at $24,250 per vial. a biotech company specializing in the development and commercialization of genetic medicines for rare diseases, announced FDA approval for Vyjuvek for the treatment of DEB.

XTalks
MARCH 8, 2023
In this episode, Ayesha talked about the FDA approval of Apellis Pharmaceuticals’ Syfovre (pegcetacoplan injection) for the treatment of geographic atrophy, a leading cause of blindness. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

XTalks
MARCH 15, 2023
Subscribe to the Xtalks Life Science Podcast to never miss a new episode. Read more here: Lenire Bimodal Neuromodulation Device by Neuromod Improves Tinnitus Symptoms in Clinical Trial The weekly podcast is available for streaming every Wednesday on Spotify , Apple Music and wherever you stream your podcasts.

XTalks
MAY 4, 2022
In this episode, Ayesha discussed the FDA approval of Mycovia Pharmaceuticals’ Vivjoa (oteseconazole) as the first approved treatment for recurrent vulvovaginal candidiasis (RVVC) or chronic yeast infection. Read the full articles here: Oteseconazole (Vivjoa) Becomes First FDA-Approved Drug for Recurrent Yeast Infection.

XTalks
DECEMBER 12, 2023
After receiving US Food and Drug Administration (FDA) approval for Fabhalta (iptacopan) last week for the treatment of the rare blood disorder paroxysmal nocturnal hemoglobinuria (PNH), Novartis presented trial data yesterday showing the drug’s promise in another indication.
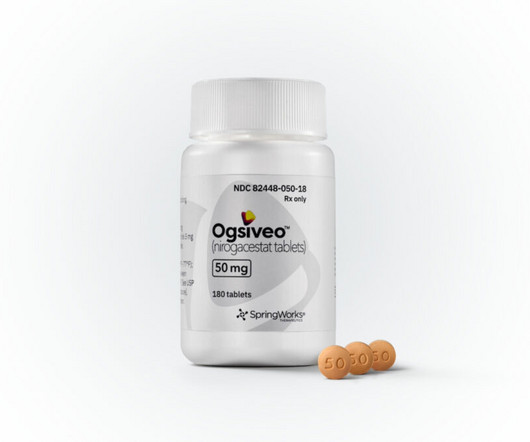
XTalks
NOVEMBER 30, 2023
Pfizer spinout SpringWorks Therapeutics’ Ogsiveo (nirogacestat) has received US Food and Drug Administration (FDA) approval for the treatment of desmoid tumors, an ultra-rare subtype of non-cancerous soft tissue sarcomas that can cause severe pain and disfigurement.

XTalks
MAY 11, 2022
Camzyos Secures FDA Approval for Obstructive HCM, Bristol Myers Eyes it as its Next Big Cardiac Drug. Subscribe to the Xtalks Life Science Podcast to never miss a new episode. Read the full articles here: Researchers Use Emerging Ovarian Cancer Biomarker to Develop New Blood Test for Ovarian Cancer.

XTalks
MAY 26, 2022
In this episode, Ayesha discussed the FDA approval of Eli Lilly’s diabetes injection Mounjaro (tirzepatide) for the treatment of adults with type 2 diabetes This is a great advancement in the diabetes space as Mounjaro is a first-in-class medicine that targets the activity of two hormones involved in hunger and blood sugar control (GLP-1 and GIP).

XTalks
DECEMBER 18, 2023
Merck received its second US Food and Drug Administration (FDA) approval for its cancer pill Welireg (belzutifan). The oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor was first approved in August 2021 for von Hippel-Lindau (VHL), a rare hereditary disease that causes tumor growth in different organs, particularly the kidney.

XTalks
MAY 25, 2023
In this episode, Vera talked about the US Food and Drug Administration’s (FDA) approval of Veozah (fezolinetant), the new type of drug for menopausal hot flashes and night sweats. Importantly, vasomotor symptoms can have a substantial impact on daily activities and overall quality of life.

XTalks
AUGUST 30, 2023
Ayesha also talked about the FDA approval of Regeneron’s high dose Eylea (HD Eylea) for the treatment of patients with wet age-related macular degeneration (wAMD), diabetic macular edema (DME) and diabetic retinopathy (DR). The states say the $13.5 Hear more about the insulin pricing controversy in this episode.
XTalks
FEBRUARY 2, 2022
In this episode, Ayesha talked about the recent FDA approval for Immunocore’s Kimmtrak for the treatment of a rare type of eye cancer called uveal melanoma. Read the full articles here: Immunocore’s Kimmtrak Gets FDA Approval as First Treatment for Rare Eye Cancer and First T Cell Receptor Therapeutic.

XTalks
JUNE 29, 2023
This episode features an interview with Marci English, Vice President and Head of BioPharma Development at Astellas Pharma, about the recent FDA approval of the company’s drug Veozah (fezolinetant) for the treatment of moderate to severe hot flashes and night sweats due to menopause.
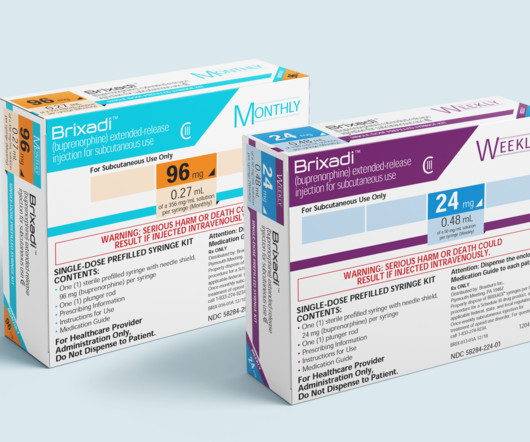
XTalks
JULY 12, 2023
Dr. Cohen talks about the recent FDA approval of Brixadi, the first long-acting buprenorphine treatment for opioid use disorder that has both weekly and monthly dosing options, and explains the technology behind its extended-release formula. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

XTalks
AUGUST 16, 2023
In this episode, Ayesha talked about the FDA approval of Zurzuvae (zuranolone) the first oral tablet for the treatment of postpartum depression (PPD). Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

pharmaphorum
NOVEMBER 4, 2020
Ireland-based Fountain Healthcare Partners has raised another €125 million ($131 million) for its third life sciences fund – 25% ahead of its target – and says it will pump most of the money into European therapeutics and medical device companies. The third fund is now closed.
XTalks
APRIL 24, 2024
ImmunityBio’s Anktiva (N-803, or nogapendekin alfa inbakicept-pmln) along with the Bacillus Calmette-Guérin (BCG) vaccine has won US Food and Drug Administration (FDA) approval for the treatment of non-muscle invasive bladder cancer (NMIBC).

XTalks
DECEMBER 15, 2021
Ayesha discusses the FDA approval of Biohaven’s intranasal spray Zavegepant for the acute treatment of migraines. Read the full articles here: Biohaven Eyes FDA Approval for Second Migraine Drug Zavegepant After Promising Trial Results. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content