Konvomep Gets FDA Approval as a New Oral Liquid Formulation Option of Omeprazole
XTalks
SEPTEMBER 6, 2022
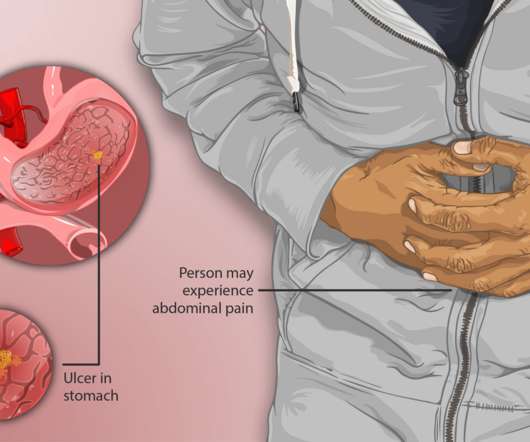
On Friday, Azurity Pharmaceuticals announced that the US Food and Drug Administration (FDA) approved their innovative oral liquid formulation Konvomep (omeprazole and sodium bicarbonate for oral suspension). John’s University College of Pharmacy & Health Sciences, Queens, New York.










































Let's personalize your content