Regeneron, Intellia target neurological diseases in expanded gene editing deal
Bio Pharma Dive
OCTOBER 3, 2023
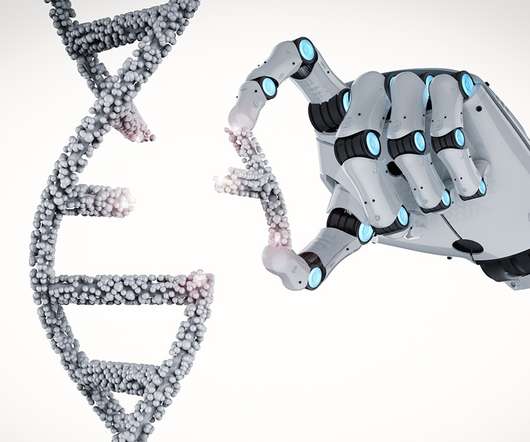
The longtime partners believe that, by combining their technologies, they can create “in vivo” genetic medicines for nervous system and muscular disorders.































Let's personalize your content