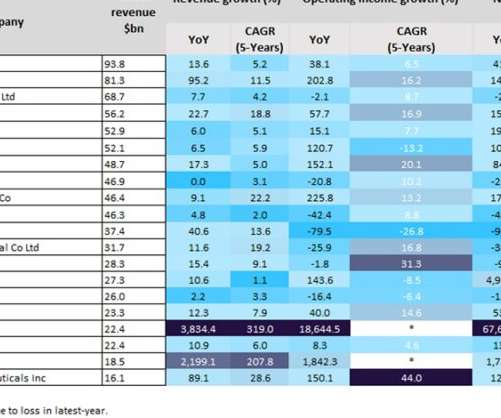
Top biopharmaceutical Covid-19 vaccine companies boosted with over 80% revenue growth
Pharmaceutical Technology
SEPTEMBER 15, 2022
BioNTech has shifted its focus towards oncology, with the company expecting the first results from a Phase II trial of its first-in-class CAR-T drug, BNT211, for multiple solid tumours. However, other challenges facing biopharmaceutical companies include growing generic drug competition, which places pressure on drug prices.















































Let's personalize your content