The drug industry continues to dare regulation
World of DTC Marketing
FEBRUARY 21, 2022
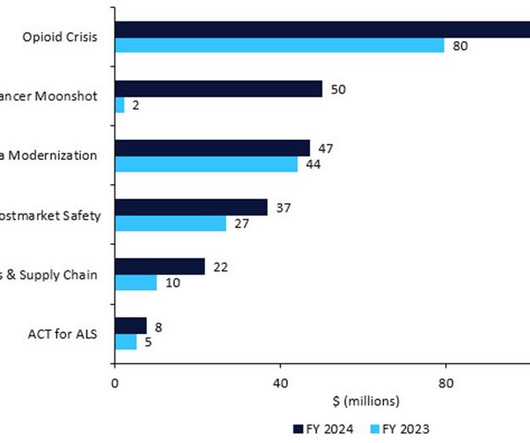
Reportedly, the cost of production for molnupiravir stands at about $17.74. The study adds further evidence to the idea that drug copay cards are a great short-term deal for patients — and especially the pharma companies that promote them — but a wrong long-term value for healthcare costs. costs about $700. spending annually.














































Let's personalize your content