FDA allows Florida to import medicines from Canada
Pharmaceutical Technology
JANUARY 8, 2024
The US FDA has granted authorisation for Florida's drug importation programme under section 804 of the FD&C Act.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 section us
section us 
Pharmaceutical Technology
JANUARY 8, 2024
The US FDA has granted authorisation for Florida's drug importation programme under section 804 of the FD&C Act.

JAMA Internal Medicine
JANUARY 23, 2024
This cross-sectional study estimates the incidence of rape-related pregnancies in US states with abortion bans.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JUNE 13, 2023
The US Food and Drug Administration (FDA) has approved Neobiosis’ investigational new drug (IND) application for ViXome to treat post-Covid-19 syndrome (also known as long Covid). ViXome is an acellular product derived from amniotic fluid. In pre-clinical testing, the therapy showed potent immunomodulatory and pro-reparative effects.

Pharma in Brief
JANUARY 7, 2024
On January 5, 2024, the US Food and Drug Administration ( FDA ) approved Florida’s proposal to import prescription drugs from Canada. This is the first approval of a Section 804 Importation Program ( SIP ) by the FDA. Prescription drugs, biologics, controlled substances, and certain other drugs (FDR section C.01.014.8).

JAMA Internal Medicine
FEBRUARY 12, 2023
This cross-sectional study determines the frequency of and rationale for US Food and Drug Administration (FDA) approval of drugs not meeting pivotal trial primary efficacy end points.

JAMA Internal Medicine
FEBRUARY 12, 2023
This cross-sectional study evaluates regulatory decisions and health technology assessments in Australia, Canada, and the UK regarding new drugs approved by the US Food and Drug Administration in 2017 through 2020, as well as estimates the US cost per patient per year for drugs receiving negative recommendations.

JAMA Internal Medicine
OCTOBER 30, 2022
This cross-sectional study estimates all US Food and Drug Administration anticancer approvals in recent years and evaluates if an association exists between their cost and efficacy.

JAMA Internal Medicine
FEBRUARY 17, 2022
This cross-sectional study examines whether an association exists between US county-level prescription rates of hydroxychloroquine and ivermectin and how people voted in the 2020 US presidential election.

JAMA Internal Medicine
JULY 2, 2023
This cross-sectional study reports trends in mortality from poisonings, firearms, and all other injuries by intent in US adults from 1999 to 2020.

ProRelix Research
JUNE 26, 2023
As per Section 201(h)(1) of the Food, Drug, and Cosmetic Act, a device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, […] The post US FDA Medical Device Applications appeared first on ProRelix Research.

JAMA Internal Medicine
JULY 4, 2022
Using national death certificate data for 2020 and provisional data for 2021 from the Centers for Disease Control and Prevention, this cross-sectional study examines the leading causes of death in the US, overall and in various age groups.

JAMA Internal Medicine
OCTOBER 8, 2023
This cross-sectional study examines whether greater periods of nursing staff turnover are associated with quality of care in US nursing homes.

JAMA Internal Medicine
APRIL 2, 2023
This cross-sectional study of data from the Surveillance, Epidemiology and End Results database assesses temporal trends in the use of active surveillance and watchful waiting vs definitive treatment in men with low- and favorable intermediate–risk prostate cancer in the US between 2010 and 2018.

STAT News
MARCH 15, 2023
Pat bade us farewell with a favorite quote from Maurice Sendak’s “Where the Wild Things Are”: “Let the wild rumpus start!” They chatted about hopes for the section, editorial pet peeves, and the vampire bats of Costa Rica.

JAMA Internal Medicine
MARCH 6, 2022
This cross-sectional study examines prostate cancer recommendations among US cancer centers to identify differences from clinical practice guidelines.

XTalks
MARCH 7, 2024
Earlier this week, the US Food and Drug Administration (FDA) took a significant step forward in regulating dietary supplements. The FDA’s decision to finalize these sections responds to feedback received on the draft and demonstrates the agency’s commitment to clarity and usability for industry stakeholders.
Cloudbyz
JULY 19, 2023
This section outlines the importance of pharmacovigilance in identifying and minimizing risks, ensuring participant safety, and maintaining the integrity of clinical trial data. This section explains the process of adverse event collection, classification, and reporting.

Pharmacy Checkers
DECEMBER 23, 2020
With Christmas upon us, I want to extoll PharmacyChecker.com’s simple core mission: make it easier for Americans to pay for their prescription drugs. Ask PharmacyChecker , the consumer journalistic section of PharmacyChecker, has a great piece up today called The Gift of Low Drug Prices. It speaks volumes about that mission.

JAMA Internal Medicine
NOVEMBER 23, 2020
This cross-sectional study examines the prevalence of persons with SARS-CoV-2 antibodies across the US and changes from July to September 2020.

JAMA Internal Medicine
FEBRUARY 14, 2021
This cross-sectional study analyzes the US Food and Drug Administration’s reasons for issuing refuse-to-file letters and assesses public transparency of refuse-to-file letters and their contents.

JAMA Internal Medicine
OCTOBER 29, 2020
This cross-sectional study examines trends in test result turnaround rates for COVID-19 testing nursing facility residents and staff in hot spot counties in the US.

JAMA Internal Medicine
AUGUST 3, 2020
This cross-sectional study examines trends in emergency department visits and visits that led to hospitalizations during a 4-month period leading up to and during the COVID-19 outbreak in the US.

JAMA Internal Medicine
JULY 13, 2020
This cross-sectional study evaluates the outcomes of universal COVID-19 testing following discovery of incident cases in 11 long-term care facilities in the US.

JAMA Internal Medicine
NOVEMBER 9, 2020
This cross-sectional study evaluates all of the novel vaccines approved by the US Food and Drug Administration over the last decade.

Cloudbyz
JULY 20, 2022
The US Food and Drug Administration has recently issued a final guidance for sponsors and applicants who receive an exemption or a waiver from providing regulatory submissions in electronic common technical document (eCTD) format. Issue Date: 24Jun2022.
Pharmaceutical Technology
APRIL 17, 2023
The US Food and Drug Administration (FDA) has approved an update to the indications and usage section of Horizon Therapeutics ’ Tepezza (teprotumumab-trbw) label to specify its use to treat thyroid eye disease (TED) patients regardless of disease activity or duration.

Pharmaceutical Technology
JUNE 5, 2023
HIPAA laws are a series of federal regulatory standards relating to the Health Insurance Portability and Accountability Act of 1996, outlining the lawful use and disclosure of protected health information in the US. Pharmaceutical companies that are publicly traded will need to pay attention,” warns Kim.

JAMA Internal Medicine
JULY 12, 2020
This cross-sectional study compares health care outcomes in middle-aged adults in the US vs England by income status.

Drug Channels
APRIL 2, 2024
For more on acquisition cost reimbursement for pharmacies, see Sections 8.4. Nonetheless, CVS Pharmacy’s cost-plus model has some notable shortcomings for plan sponsors and is far less “disruptive” than the company would like us to believe. and 12.3.4. of our new 2024 Economic Report on U.S. Pharmacies and Pharmacy Benefit Managers.
Cloudbyz
MAY 22, 2020
There are general templates that may be used to construct a protocol. Typically, a study protocol has the following sections: Note: This ordering is general and can be rearranged to what appropriately fits your study. 1) Protocol Summary : This section is a synopsis of the study protocol document. TABLE OF CONTENTS: —.

Drug Channels
OCTOBER 10, 2023
The notable new material in this 2023-24 edition includes: New data about commercial pricing and reimbursement for provider-administered biosimilars appears in Section 3.2.2. and Section 6.4.3. A new Section 6.2.2. A new Section 6.3.2. Group purchasing organizations for small pharmacies (Section 2.2.5.)

Camargo
MARCH 25, 2021
The Food and Drug Administration Safety and Innovation Act (FDASIA) of 2012 added Section 529 to the FD&C Act , establishing the RPDD program and its associated priority review vouchers. It must also be a rare disease or condition as described in the FD&C Act, with a prevalence of less than 200,000 people in the US.

FDA Law Blog
JANUARY 7, 2024
Cato — New Year’s is often associated with baby New Year and with resolutions, which in a convoluted way got us thinking about post-approval pregnancy studies. Lots of us start the new year with a resolution. To assess signals of serious risk related to the use of the drug.” By McKenzie E.

FDA Law Blog
APRIL 21, 2024
Government—was that, despite having missed the statutory 60-day filing deadline, the patent was granted a PTE due to a change in the law created by Section 37 of the Leahy-Smith America Invents Act (“AIA”) ( Pub. which brings us to the topic of this post. sections 331(a) and 352.(a)(l), Taiho Pharmaceutical Co.,

Pharmacy Checkers
MARCH 5, 2021
I reviewed the drug relabeling section of Florida’s Drug Importation Program submission to the U.S. Florida shows us: The standard U.S. It’s called parallel trade; it results in price competition – and that’s what states are doing with Section 804. Department of Health and Human Services, and it really tells a story.
pharmaphorum
NOVEMBER 23, 2020
German digital health company smartpatient is adding a new section of its MyTherapy app providing information on wet age-related macular degeneration (AMD), a leading cause of blindness. Novartis earned $2 billion from Lucentis last year, while Roche – which sells it in the US – made $1.8 billion from the drug. billion.

Drug Discovery World
MARCH 31, 2023
The US Food and Drug Administration (FDA) has granted Fast Track Designation to RRx-001 for the prevention/attenuation of severe oral mucositis in chemotherapy and radiation-treated head and neck cancer patients. The post US FDA fast-tracks drug to prevent severe chemotherapy side effect appeared first on Drug Discovery World (DDW).

The Pharma Data
JUNE 12, 2021
Your use of the information on this site is subject to the terms of our Legal Notice. You should view the News section and the most recent SEC Filings in the Investor section in order to receive the most current information made available by Johnson & Johnson Services, Inc. Do Not Sell My Personal Information.

The Pharma Data
MAY 7, 2021
Your use of the information on this site is subject to the terms of our Legal Notice. You should view the News section and the most recent SEC Filings in the Investor section in order to receive the most current information made available by Johnson & Johnson Services, Inc. Do Not Sell My Personal Information.
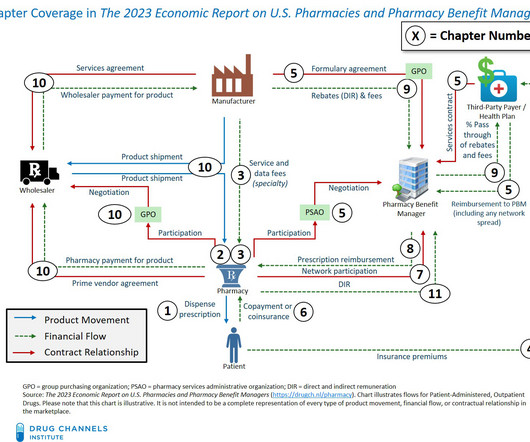
Drug Channels
MARCH 14, 2023
The report has been thoroughly updated with the latest data and includes loads of new material and new sections. Please contact us if you did not receive the email. Pharmacies and Pharmacy Benefit Managers , our 14th edition, provides a comprehensive, fact-based, and non-partisan tool for understanding the entire U.S.

Drug Channels
OCTOBER 5, 2021
The notable new material in this 2021-22 edition includes three new sections: Section 4.2.5. Section 4.4. Section 7.4.4. We consider McKesson’s exit from Europe in Section 9.4.3.) (We consider McKesson’s exit from Europe in Section 9.4.3.) Section 6.1.5. WHAT’S GOING ON.

Camargo
MAY 19, 2021
To obtain HUD classification, a device must meet several criteria: It must be intended to benefit patients in the treatment or diagnosis of a rare disease or condition that affects or is manifested in 8,000 or fewer people in the US per year or is a rare subset of a non-rare disease or condition. Timeline and Maintenance.

FDA Law Blog
MAY 3, 2023
He later served as Staff Coordinator in Diversion Control’s Liaison and Policy Section at DEA headquarters. We hope that you will be able to join us! Houck conducted numerous scheduled cyclic and targeted inspections and investigations as a DEA Diversion Investigator in the field for ten years.

Drug Discovery World
NOVEMBER 30, 2023
As with all gene therapy products with integrating vectors (lentiviral or retroviral vectors), the potential risk of developing secondary malignancies is labelled as a class warning in the US prescribing information (USPIs) for approved BCMA-directed and CD19-directed genetically modified autologous T cell immunotherapies.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content