Cartherics grants licence for CTH-004 to Shunxi
Pharmaceutical Technology
MAY 1, 2023
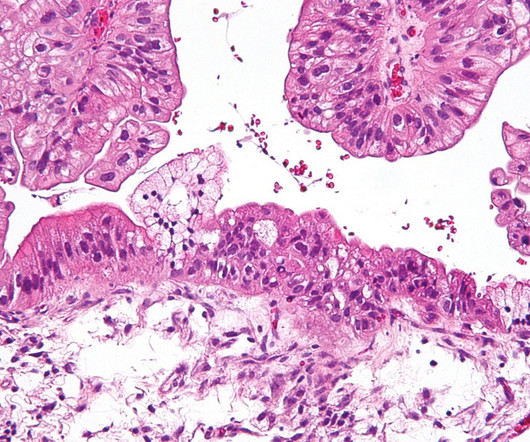
Under the deal terms, Shunxi will have licence to develop, manufacture, and commercialise CTH-004 to treat several solid tumours including ovarian cancer in Greater China, including Hong Kong, Taiwan, Mainland China, and Macao. In animal models of ovarian cancer, it demonstrated promising results.






































Let's personalize your content