CRISPR tagging improves accuracy of model cells grown from stem cells
Scienmag
DECEMBER 1, 2020
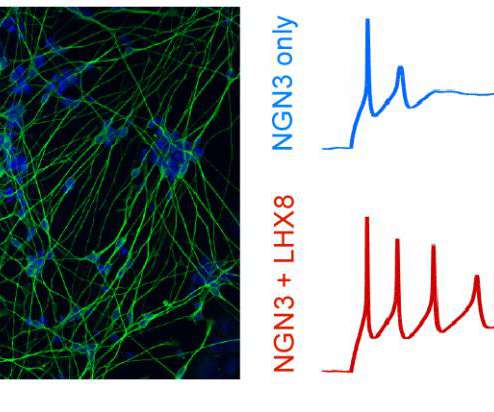
Tagging produces detailed catalog of transcription factors key to making each cell type Credit: Gersbach Lab, Duke University DURHAM, N.C. – A team of biomedical engineers at Duke University has created a new way to turn stem cells into a desired cell type by mastering the language of gene regulatory networks.

























Let's personalize your content