Smart pharmaceutical and healthcare labels: Lets trace medicines from its origin
Roots Analysis
MARCH 8, 2023
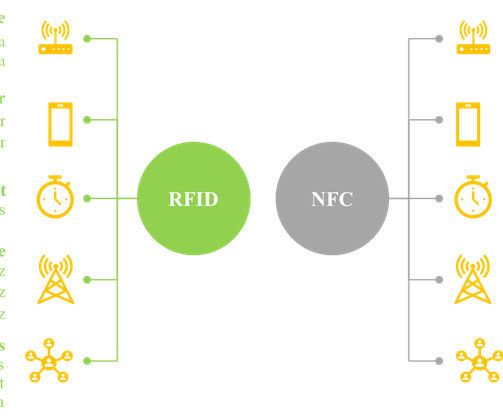
Traditionally, text and images were printed on pharmaceutical products to convey important information. It is worth noting that smart labels contain a transponder code which can be read by sophisticated devices, including radio frequency identification device (RFID) tags and near-field communication (NFC) chips.





















Let's personalize your content