Big numbers blur policy ambitions
Scienmag
JULY 4, 2022
Government policies often are presented with hefty price tags, but people often zone out as more zeros are added to the total cost.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag policy
tag policy 
Scienmag
JULY 4, 2022
Government policies often are presented with hefty price tags, but people often zone out as more zeros are added to the total cost.

Pharma Tutor
DECEMBER 7, 2021
Pharmaceutical Education Policy. Read more about Pharmaceutical Education Policy Log in or register to post comments About Author. Chief Editor. Journal of Pharmaceutical Research. Krupanidhi College of Pharmacy. Bengaluru-560035. Tue, 12/07/2021 - 17:15.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
NOVEMBER 16, 2022
As one of the federal departments and agencies with the largest research and development budget, the NIH came out with one of the first federal data sharing policies in 2003 called the NIH Data Sharing Policy and Implementation Guidance. pctGR, @Collaboratory1.

Rethinking Clinical Trials
APRIL 27, 2023
The MyTEMP trial is a pragmatic, cluster randomized controlled trial in Ontario, Canada, to determine if adopting a default center-wide policy of personalized cooler dialysate is superior to a standard temperature dialysate of 36.5 Cluster randomized trials of hemodialysis center-wide policies raise complex ethical issues.

Rethinking Clinical Trials
FEBRUARY 7, 2024
Consumers trust policy – and the scientific evidence on which it is based – if communicated to them properly. Sound science, sound policy, and sound communication are each fundamental to the Agency’s success.
Pharmaceutical Technology
DECEMBER 5, 2022
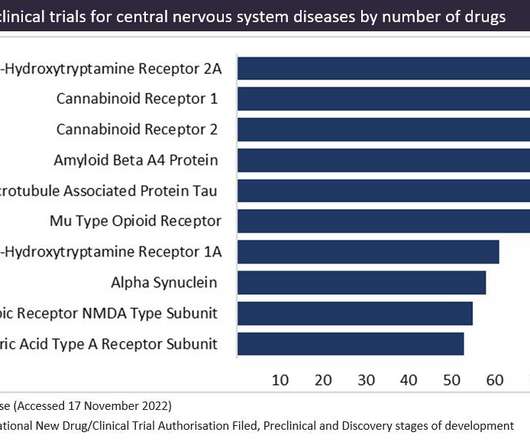
Collectively, cannabinoid receptors (CB1 and CB2) are currently the most popular targets in preclinical stage of development, with 391 drugs tagged in total. Strict policies in Asia, the Middle East and Africa could lead to restricted progress in late-stage development, which could impact the future market.

Rethinking Clinical Trials
JANUARY 17, 2024
The COVID Therapeutics Committee worked with the state health department to develop a policy for fair allocation of scarce medications to treat COVID-19. Tags #pctGR, @Collaboratory1 The post Grand Rounds January 12, 2024: Design and Implementation of a Weighted Lottery to Equitably Allocate Scarce Covid-19 Resources (Erin K.
Pharmaceutical Technology
MAY 1, 2023
CTH-004 is developed by genetically altering patient T cells for inserting a chimeric antigen receptor (CAR) to target a marker (TAG-72) on ovarian cancer cells and delete genes which are involved in T cell function suppression. Shunxi will also hold an option to negotiate rights to other CAR-T products, which include the licenced IP.

Rethinking Clinical Trials
OCTOBER 26, 2023
What are the policy considerations to get to easier testing? Tags #pctGR, @Collaboratory1 The post Grand Rounds October 20, 2023: A National Initiative to Eliminate Hepatitis C in the United States – Why This Matters to Clinical Trialists (Rachael L. Fleurence, PhD, MSc Senior Advisor National Institutes of Health Joshua M.

Rethinking Clinical Trials
JUNE 29, 2022
Office of Medical Policy (OMP). ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ?. Speaker. John Concato, MD, MS, MPH. Associate Director for Real-World Evidence Analytics. Center for Drug Evaluation and Research (CDER). Food and Drug Administration (FDA). and the FDA Draft Guidance for RWD/RWE. pctGR, @Collaboratory1.

The Pharma Data
MAY 7, 2021
This site uses cookies as described in our Cookie Policy. Remarketing tags may not be associated with personally identifiable information or placed on pages related to sensitive categories. See more information and instructions on how to setup the tag on: [link] >. You are now leaving jnj.com.

Rethinking Clinical Trials
DECEMBER 20, 2023
Tags #pctGR, @Collaboratory1 The post Grand Rounds December 15, 2023: Diversifying Clinical Trials: A Path Forward (Roxana Mehran, MD, FACC, FAHA, MSCAI, FESC) appeared first on Rethinking Clinical Trials. With the current ability to become pregnant this can affect women up to the age of 50.

Rethinking Clinical Trials
OCTOBER 27, 2022
In a recent paper, Health advertising on Facebook: Privacy and Policy Considerations, patients download their raw data, and found who was tracking them across the internet. Three out of 5 companies included in the study were not adhering to their privacy policies. Health advertising on Facebook: Privacy and policy considerations.

The Pharma Data
AUGUST 17, 2020
This site uses cookies as described in our Cookie Policy. Remarketing tags may not be associated with personally identifiable information or placed on pages related to sensitive categories. See more information and instructions on how to setup the tag on: [link] >. You are now leaving jnj.com.

The Pharma Data
AUGUST 14, 2020
This site uses cookies as described in our Cookie Policy. Remarketing tags may not be associated with personally identifiable information or placed on pages related to sensitive categories. See more information and instructions on how to setup the tag on: [link] >. You are now leaving jnj.com.

The Pharma Data
AUGUST 20, 2020
This site uses cookies as described in our Cookie Policy. Remarketing tags may not be associated with personally identifiable information or placed on pages related to sensitive categories. See more information and instructions on how to setup the tag on: [link] >. You are now leaving jnj.com.

Rethinking Clinical Trials
JANUARY 19, 2023
Tags #pctGR, @Collaboratory1 The post Grand Rounds Ethics and Regulatory Series January 13, 2023: Ethical Considerations When Vulnerable Populations are Subjects in Pragmatic Trials (Emily A. Speakers Emily A. That’s difficult. it can be a barrier.

The Pharma Data
MAY 3, 2021
This site uses cookies as described in our Cookie Policy. Remarketing tags may not be associated with personally identifiable information or placed on pages related to sensitive categories. See more information and instructions on how to setup the tag on: [link] >. You are now leaving jnj.com.

Rethinking Clinical Trials
MAY 25, 2023
The pragmatic randomized control trial was designed from a hospital policy perspective. Tags #pctGR, @Collaboratory1 The post Grand Rounds May 19, 2023: Aspirin or Low-Molecular-Weight Heparin for Thromboprophylaxis After a Fracture (Robert O’Toole, MD) appeared first on Rethinking Clinical Trials.

Pharmaceutical Technology
APRIL 13, 2023
Although the findings in the report are only preliminary, they shed light at the considerations behind the high price tags of gene therapies. Sickle cell disease is an inherited blood disorder that is caused by mutations in the HBB gene, which codes for the oxygen-carrying protein haemoglobin in red blood cells.

pharmaphorum
JANUARY 21, 2022
Access to medicines is “one of the most challenging policy areas in every country in the European region” – it’s time to develop a solution that works for everyone. In recent years, revolutionary medicines have come with price tags to match, but this is simply a symptom of our current model.

Rethinking Clinical Trials
JULY 6, 2023
Interstate licensing and our own institution’s policies are issues that still need to be addressed in terms of oversight and responsibilities. In some ways, health care providers are the same as any other data instrument in the trial – they are external data sources that come back to the investigator for safety and quality review.

Rethinking Clinical Trials
DECEMBER 14, 2022
The policy level point is well taken; saying that they are prohibited might be too strong, but you do need a justification or rationale for stepped wedge design, especially over parallel. As a statistician, I agree but it’s important to do the extra work and look at alternative designs. pctGR, @Collaboratory1.

Rethinking Clinical Trials
SEPTEMBER 16, 2022
Department of Medical Ethics & Health Policy. Steven Joffe, MD, MPH. Art and Ilene Penn Professor and Chair. University of Pennsylvania Perelman School of Medicine. Ethics, Learning Health System. Key Points. It is incumbent on all of us, and it should be a central part of the goal for all of us to deliver care to patients and families.

XTalks
JUNE 28, 2023
million price tag of Elevidys, a one-time gene therapy. Ingram expects the launch to take a few months to pick up due to logistical and policy issues. It has taken Sarepta six years to develop Elevidys with several roadblocks in its journey, including two clinical holds due to safety concerns.

Rethinking Clinical Trials
AUGUST 28, 2023
Speakers Prof Sir Martin Landray, FMedSci Professor of Medicine & Epidemiology University of Oxford Chief Executive, Protas Khair ElZarrad, PhD, MPH Director, Office of Medical Policy Center for Drug Evaluation and Research (CDER) U.S. One important note is that we specifically moved away from a checklist approach.

Rethinking Clinical Trials
AUGUST 28, 2023
Speakers Prof Sir Martin Landray, FMedSci Professor of Medicine & Epidemiology University of Oxford Chief Executive, Protas Khair ElZarrad, PhD, MPH Director, Office of Medical Policy Center for Drug Evaluation and Research (CDER) U.S. One important note is that we specifically moved away from a checklist approach.

pharmaphorum
SEPTEMBER 29, 2021
Employers can monitor electronic communication, and people have been fired for violating related policies. Violation of email policy and messages containing inappropriate or offensive language are the two most common causes for email-related firing in the US. Know your organisation’s policies.

Pharmaceutical Technology
APRIL 5, 2023
But access to these treatments continues to remain limited due to high price tags and variable availability across regions. By Cytiva Thematic By downloading this case study, you acknowledge that GlobalData may share your information with Cytiva Thematic and that your personal data will be used as described in their Privacy Policy

Pharmaceutical Technology
JUNE 21, 2023
The high price tag may be hard to defend , but with lifetime treatment costs for haemophilia B reaching as high as $23m for some, the single-dose treatment could prove far more cost-effective. Despite the limited market, the price tags of haemophilia make it potentially lucrative. Haemophilia B is a rare condition.

Delveinsight
JULY 22, 2020
The hospital inventory is equipped with tagged sensors. Moreover, the opportunities in the market has led to an increase in the number of collaboration and Alliances between Tech companies and healthcare providers, IoT based insurance policies are also expected to have a positive impact on the growth of the IoT in the healthcare market.

XTalks
FEBRUARY 27, 2024
Rare Disease Day 2024, which falls on February 29 this year, is an opportunity to unite under a common cause: to bring attention to the challenges faced by those living with rare diseases and to push for advancements in research, treatment and policy. Sarepta hopes to clarify Elvidys’ effectiveness in older children in a confirmatory trial.

The Pharma Data
MAY 15, 2021
This is the Privacy Policy for PentagonFit and this Website www.pentagonfit.com (individually and collectively the “Company”). When you use this Website, you agree to the Privacy Policy that follows. If you do not agree with this Privacy Policy you should immediately stop using this Website. Changes to Privacy Policy.

Cloudbyz
JUNE 1, 2023
Organizations can manage their encryption keys and set encryption policies. Transaction Security: Transaction Security gives real-time security policy enforcement based on user behavior. It allows businesses to define, enforce, and monitor data access and usage policies in real time.

Pharmaceutical Technology
MARCH 17, 2023
In some cases, she said, “[drug] innovation would have been otherwise hindered by their price tag alone”. Watt explained that for newer, first-in-class drugs these types of agreements can also give healthcare systems safety in their decisions to approve certain drugs. Click here to watch this webinar and access the presentation.

The Pharma Data
MAY 4, 2021
Guidance for Adjusted other (income)/deductions (2) includes an estimated benefit of approximately $300 million resulting from a change in accounting principle to a more preferable policy under U.S. This change went into effect in the first quarter of 2021 and prior period amounts have been recast to conform to the new accounting policy. (4)

Pharmaceutical Technology
NOVEMBER 30, 2022
The company’s weight loss drug Saxenda (liraglutide) won an approval for chronic weight management in December 2014, and Wegovy (semaglude) got the same tag in 2021. By clicking the Download Free Report button, you accept the terms and conditions and acknowledge that your data will be used as described in the GlobalData privacy policy.

Pharmaceutical Technology
NOVEMBER 30, 2022
The company’s weight loss drug Saxenda (liraglutide) won an approval for chronic weight management in December 2014, and Wegovy (semaglude) got the same tag in 2021. By clicking the Download Free Report button, you accept the terms and conditions and acknowledge that your data will be used as described in the GlobalData privacy policy.

XTalks
FEBRUARY 14, 2023
The 2023 Farm Bill is the comprehensive package that US Congress uses to set national agriculture, conservation, nutrition and forestry policy. Spending on the nutrition title alone is expected to push the price tag for the next Farm Bill to more than $1 trillion over the next ten years.

World of DTC Marketing
JUNE 16, 2021
The pharma industry has been riding a wave of public approval since they developed a vaccine to fight COVID-19, but that goodwill is going to be short lived thanks, in part, to the $56,000 price tag of Biogen’s new drug and insulin that too many people still can’t afford.

Pharma Marketing Network
JUNE 8, 2020
I mean, you know, there has obviously been a lot of breakthrough biologics and different therapeutics like around cancers and things like that, that have high price tags because maybe they don’t have as big a market as you know, something’s wrong, you know blood pressure medication. So yeah, there is plenty to talk about there.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content