FDA issues Cipla warning letter after thousands of complaints
Pharmaceutical Technology
NOVEMBER 22, 2023
The warning letter issued to Cipla will further delay the launch timelines of the company’s Advair generic in the US.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 warning-letters
warning-letters 
Pharmaceutical Technology
NOVEMBER 22, 2023
The warning letter issued to Cipla will further delay the launch timelines of the company’s Advair generic in the US.

Fierce Pharma
APRIL 10, 2024
In a four-observation warning letter issued this week, the U.S. It usually goes without saying that your pharmaceutical production workers need to be gowned and gloved while handling drug materials inside clean rooms. |
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
FEBRUARY 14, 2024
FDA unleashes multiple warning letters targeting insanitary manufacturing and online sales of unapproved Mounjaro, Ozempic fkansteiner Wed, 02/14/2024 - 05:10

BioSpace
NOVEMBER 29, 2023
The troubled Indian pharma company received its second FDA warning letter in months, which this time cited quality control and data integrity lapses at its manufacturing facility in Gujarat, India.

Fierce Pharma
SEPTEMBER 12, 2023
Eight companies are finding themselves in hot water for allegedly churning out illicit eye drugs, the FDA said Tuesday. | The companies included in the warning letter sweep are: Boiron, CVS Health, DR Vitamin Solutions, Natural Ophthalmics, OcluMed, Similasan, TRP Company and Walgreens Boots Alliance.

FDA Law Blog
JANUARY 1, 2024
Maybe that’s also why FDA last week publicized the highest number of important Warning Letters of the year (compared with prior releases in 2023). Warning Letters, generally made public in a batch each Tuesday, are FDA’s public sanction that is most widely used to bring pressure on manufacturers and clinical trial investigators.

XTalks
SEPTEMBER 19, 2023
The US Food and Drug Administration (FDA) has issued warning letters to eight companies manufacturing or marketing unapproved eye products that violate federal law. The FDA said the warning letters are part of the agency’s ongoing effort to protect Americans from potentially harmful ophthalmic products.

Fierce Pharma
AUGUST 2, 2023
Intas Pharmaceuticals’ new warning letter from the U.S. FDA reads like a checklist of what not to do when the regulator pays a visit to your manufacturing facility. | The FDA handed Intas a five-observation write up after inspecting the company’s Sanand, India, production plant from November 22 to December 2, 2022.

XTalks
JANUARY 31, 2024
The US Food and Drug Administration (FDA) has issued letters to several companies to include a class-wide boxed warning about the risk of T-cell malignancies on their CAR T-cell therapies. The FDA sent each company individual letters on January 19 outlining the label update requirement.

XTalks
NOVEMBER 24, 2022
Earlier this week, the US Food and Drug Administration (FDA) sent out warning letters to five companies illegally selling CBD-infused food and beverage products. Perhaps warning letters were sent out to these five companies since four of them sell CBD-infused candies or gummies. 331(ll) and 331(a).”.

BioSpace
FEBRUARY 7, 2023
regulator issued a warning letter to India’s Intas Pharmaceuticals regarding violations of current good manufacturing practice. Following reports of destroyed and discarded data, the U.S.

FDA Law Blog
AUGUST 10, 2023
Cato — On August 3rd, FDA issued 11 warning letters to foreign facilities registered as OTC drug manufacturers. For each of these facilities, FDA did not conduct an on-site inspection of the facility prior to issuing the warning letter. By McKenzie E.

Pharmaceutical Technology
FEBRUARY 14, 2024
The FDA has issued warning letters to two online sellers for selling unapproved versions of semaglutide and tirzepatide.

XTalks
DECEMBER 2, 2022
Last week, the US Food and Drug Administration (FDA) sent out warning letters to five companies illegally selling CBD-infused food and beverage products. In this episode of the Xtalks Food Podcast, Sydney talks about the contents of the warning letters and the FDA’s rules regarding CBD.

FDA Law Blog
SEPTEMBER 6, 2023
“Commits” is probably not the right word, although it is a term repeated frequently in the Guidance, since the Guidance at this point is only a draft, and, like other FDA guidances, it states in a highlighted warning that it “does not establish any rights for any person and is not binding on FDA or the public.”

FDA Law Blog
MAY 23, 2022
By Riëtte van Laack — On Monday May 9, 2022, FDA issued an “ update ” announcing that it had sent 11 Warning Letters to companies that distributed dietary supplements alleged to be adulterated because one or more ingredients in the companies’ products contained an illegal dietary ingredient.

FDA Law Blog
AUGUST 14, 2023
One inspection resulted in FDA’s securing a consent decree to restrict or shut down operations, and two resulted in an “untitled letter,” which is not available to the public on FDA’s website. OAI is FDA’s classification for facilities it deems to be in an unacceptable state of compliance.
Eye on FDA
AUGUST 16, 2023
This past June FDA posted an Untitled Letter , the first regulatory action letter in a year, reported on here regarding a website communication. This month FDA posted another letter , this one a Warning Letter involving a Sales Aid. Future letters will indicate if this persists as a point of interest.

Fierce Pharma
JANUARY 24, 2024
The FDA’s letter demanding a labeling change for Gilead Sciences’ Tecartus temporarily went missing on the agency’s website Tuesday. | The FDA’s letter demanding a labeling change for Gilead Sciences’ Tecartus temporarily went missing on the agency’s website Tuesday. Instead, it's adjusted the wording of a proposed boxed warning.

BioSpace
OCTOBER 27, 2021
The letter was related to a 2021 FDA inspection connected to Sesen’s Biologics License Application for Vicineum for the treatment of BCG-unresponsive non-muscle invasive bladder cancer.
Eye on FDA
JANUARY 26, 2022
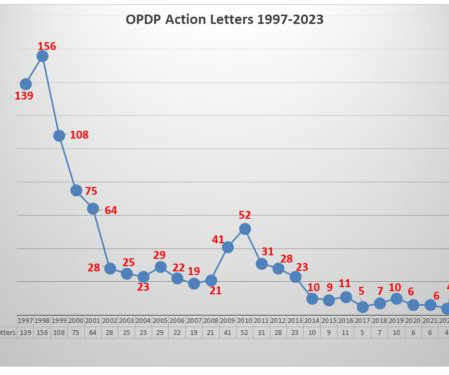
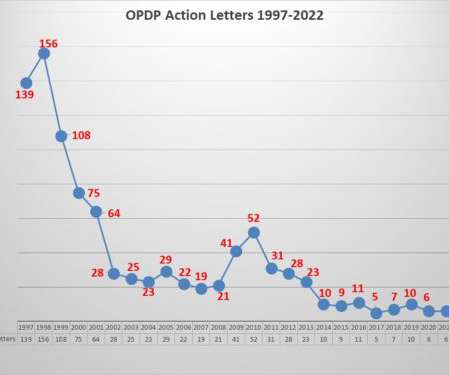
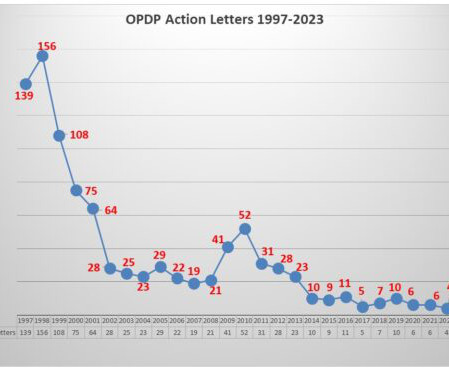
FDA’s Office of Prescription Drug Promotion (OPDP) issued the first regulatory action letter for 2022. Another characteristic of recent enforcement actions over the past several years is that smaller, less well-known companies have mostly been the recipients of letters from OPDP. This one has some notable characteristics.

Fierce Pharma
SEPTEMBER 20, 2023
After a manufacturing-related complete response letter held up the U.S. | But now the company warned a decision will likely be delayed past the third quarter. UCB had previously expected an FDA decision during the first half of the year after suffering a prior rejection on its medicine.

FDA Law Blog
APRIL 11, 2022
A December 2021 warning letter to Medtronic, Inc. Medtronic Warning Letter) provides an important reminder from FDA as to the correct estimation of risk associated with malfunctions. This estimation of risk is important because it drives both decision making and regulatory compliance.
Eye on FDA
AUGUST 22, 2023
Last week, a Warning Letter was posted regarding a sales aid. This week it action came in the form of an Untitled Letter (NOV) sent to a company regarding a paid social media posting. Enforcement this year was non-existent until June when the agency posted its first letter in a year. Pharma communicators take note.
FDA Law Blog
JANUARY 22, 2024
OC also has input into FDA’s more forceful enforcement measures, like warning letters, import alerts, and consent decrees. Recalls, warning letters to online pharmacies, and drug-related import alerts were key tools for OC’s efforts to safeguard the drug supply chain from adulterated products over the prior fiscal year.

FDA Law Blog
AUGUST 16, 2023
He talked about regulatory discretion being exercised for drug manufacturing facilities with serious compliance problems, about how firms should respond to FDA inspectional observations, and about an upcoming guidance that will be of interest to generic drug manufacturing firms that have received Warning Letters.
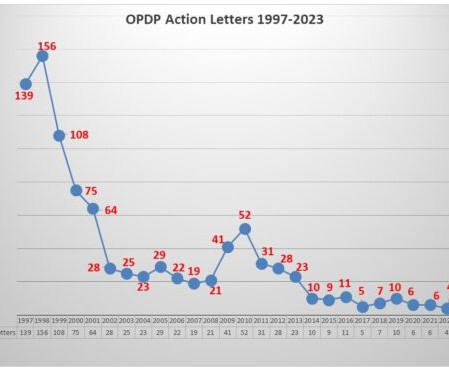
Eye on FDA
NOVEMBER 9, 2023
While enforcement has been at a low ebb for quite some time with FDA’s Office of Prescription Drug Promotion (OPDP), this week took a different turn with the posting of two new untitled letters sent October 31. There was no concentration among products with boxed warnings. But there has been one trend worth noting.

XTalks
JANUARY 12, 2021
The US Food and Drug Administration (FDA) has sent a warning letter to Coco’s Holistic Specialties & Apothecary , an online holistic and Eastern medicine company, for falsely advertising its products’ ability to prevent, treat, cure and even diagnose people with COVID-19. 352 for false or misleading labeling.
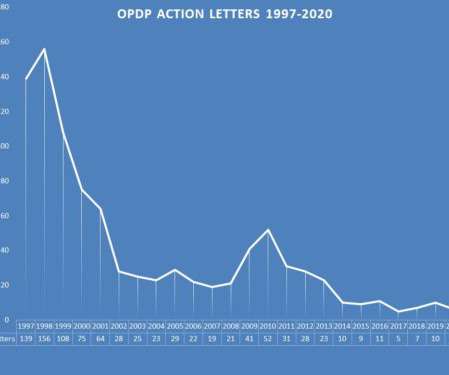
Eye on FDA
FEBRUARY 10, 2021
And as in recent years, the regulatory action letters were exclusively aimed at smaller, less experienced companies. The collection of letters issued by OPDP during 2020 can be found here. Warning Letters are the more serious of the two types of letters sent by the agency. So when OPDP did act, they meant business.

STAT News
JANUARY 12, 2023
Every few months, we’re warned that the Omicron variant of the SARS-CoV-2 virus has spawned yet another subvariant, this one even more transmissible than the ones it is fast overtaking. The new entity is given a name, an unwieldy string of letters and numbers separated by periods. It’s like clockwork now.

Pharmaceutical Commerce
AUGUST 17, 2023
The act is currently set to go into effect in just over three months.
BioPharma Reporter
AUGUST 4, 2020
Takeda aims to have the plant that manufactures drugs including Entyvio and leuprorelin ready for re-inspection within 12 months.

STAT News
JANUARY 10, 2023
Food and Drug Administration for a host of serious manufacturing violations at a key plant in India, the latest instance in which the company was tagged by the regulator for quality-control problems.
STAT News
NOVEMBER 18, 2022
A FDA official says that most warning letters issued for manufacturing issues in fiscal year 2022 were the result of onsite inspections, reversing a pandemic-era trend of enforcement actions triggered by the use of alternative tools , according to Regulatory Focus. But the agency still faces a backlog.

XTalks
NOVEMBER 20, 2023
Last week, the FTC issued warning letters to two major groups in the food and beverage sector and several online food industry influencers. Food industry influencers were recently at the center of a controversy highlighted by the Federal Trade Commission (FTC). Their offense?

ACRP blog
FEBRUARY 5, 2024
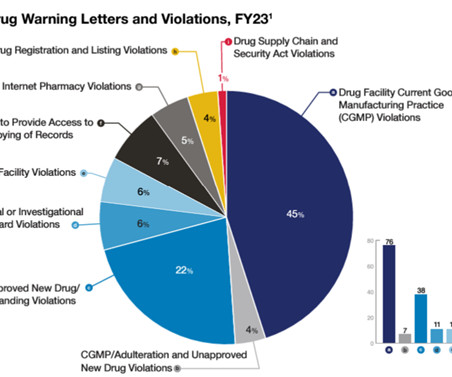
Between 2017 and 2022, FDA issued more than 160 Warning Letters citing data integrity deficiencies,{1} which relate to the completeness, consistency, and accuracy of data.{2} Food and Drug Administration (FDA) investigators, leading to a range of regulatory actions.
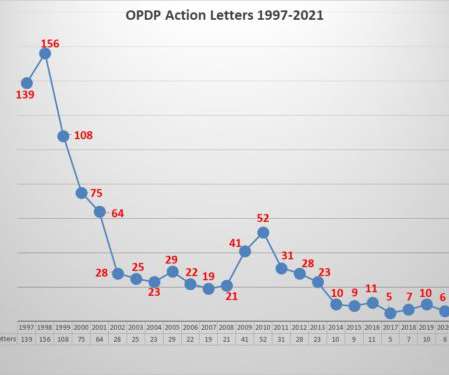
Eye on FDA
JANUARY 9, 2022
This year there were 5 regulatory action letters issued by OPDP. This year there two were Warning Letters and three were Untitled Letters. The 5 letters issued involved 8 different communications vehicles for a total of 9 violations that were cited by the agency as reasons for the action.

XTalks
NOVEMBER 29, 2023
The US Food and Drug Administration (FDA) is warning about rare but serious side effects for the antiseizure drugs levetiracetam (Keppra, Keppra XR, Elepsia XR, Spritam) and clobazam (Onfi, Sympazan). The FDA is requiring that warnings about DRESS be added to the prescribing information and patient Medication Guides for the antiseizure drugs.
XTalks
SEPTEMBER 23, 2022
The US Food and Drug Administration (FDA) issued a stern warning to address a disturbing trend among teens: cooking chicken in NyQuil. One social media trend relying on peer pressure is online video clips of people misusing nonprescription medications and encouraging viewers to do so too,” the FDA stated in the warning letter.

FDA Law Blog
MARCH 11, 2024
Mullen — On February 20, 2024, FDA issued a letter to the medical device industry ( link ) warning medical device firms of recent FDA concerns related to fraudulent and unreliable laboratory testing data in premarket submissions. Unfortunately, the letter provides little new information to guide industry conduct.

FDA Law Blog
JANUARY 3, 2024
To carry out its mission, CVM has the same enforcement tools that the rest of FDA, like CDER and CDRH, have, including the authority to conduct inspections, issue warning letters, seize products, add manufacturers to import alerts, and impose penalties on bad actors. And CVM certainly exercises that authority.

Eye on FDA
MARCH 10, 2021
OPDP Warning Letter Change. Not as compelling perhaps as Harry and Meghan splitting from Buckingham Palace, but there has been a split at FDA’s OPDP between Warning and Untitled letters. There is a drop down menu that includes all of the various points of origin for a Warning Letter within FDA.

FDA Law Blog
AUGUST 16, 2023
In what may be the surprise “ Warning Letter of the Year ” (can you tell this blogger was pretty shocked?) Why is this Warning Letter so shocking? Let’s take a walk down OPDP Warning Letter memory lane. Let’s take a walk down OPDP Warning Letter memory lane. Pretty egregious stuff, right?

The Pharma Data
JANUARY 20, 2021
You can count on Drug Industry Daily for insightful, accurate articles supported by links to additional key documents, such as FDA guidances and comments, warning letters, full texts of proposed legislation, Federal Register postings, GAO reports and more. There’s absolutely no risk to you.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content