Antibodies, Immunity, and COVID-19
JAMA Internal Medicine
NOVEMBER 23, 2020
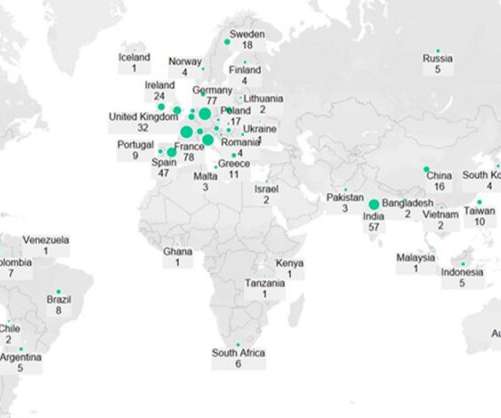
Widespread availability of commercial assays that detect anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies has enabled researchers to examine naturally acquired immunity to coronavirus disease 2019 (COVID-19) at the population level.



































Let's personalize your content