FDA approves Alentis’ IND for ALE.C04 to treat CLDN1+ tumours
Pharmaceutical Technology
JUNE 15, 2023
ALE.C04 is an investigational antibody designed to target exposed CLDN1 in cancer cells. This approval has been granted for ALE.C04 both as a monotherapy and in combination with pembrolizumab in a first-in-human clinical study in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC).











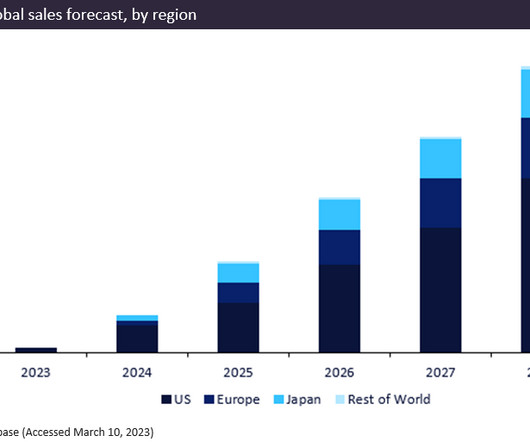

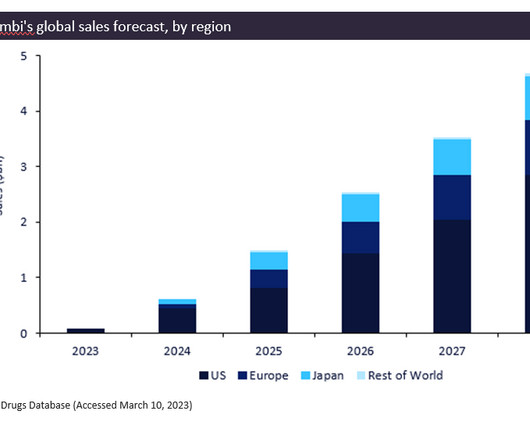





















Let's personalize your content