Cell therapy enables insulin independence without immunosuppression
Drug Discovery World
FEBRUARY 19, 2024
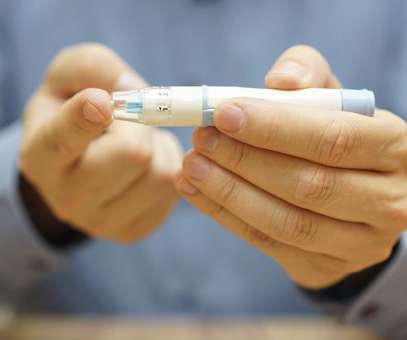
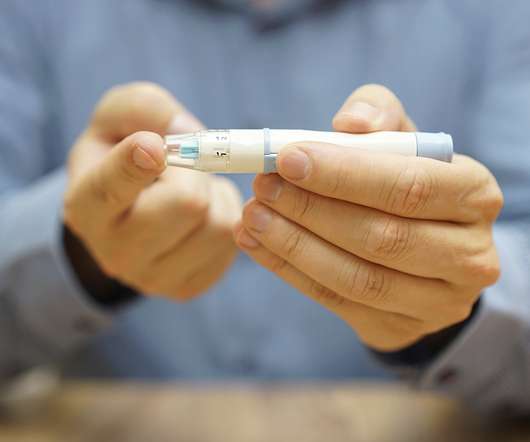
Preclinical data has demonstrated insulin independence following transplantation of hypoimmune (HIP) allogeneic primary islet cells without immunosuppression in a diabetic non-human primate (NHP). Following this, blood glucose levels in the diabetic NHP began to fluctuate and increase markedly, and insulin injections needed to be resumed.




































Let's personalize your content