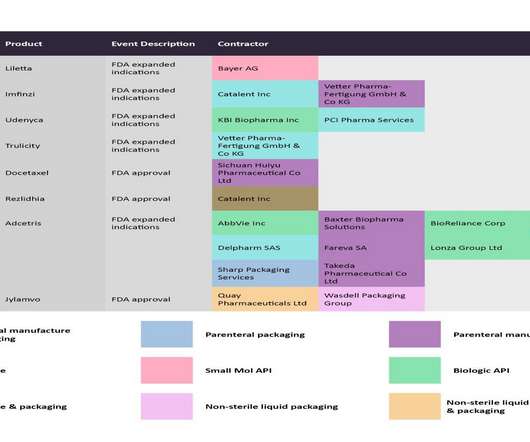
GSK details Blenrep combo data that could bring the multiple myeloma ADC back to life
Fierce Pharma
FEBRUARY 5, 2024
If a new package of pivotal data satisfies the FDA, GSK could have another run at the U.S. | If a new package of pivotal data satisfies the FDA, GSK could have another run at the U.S. multiple myeloma market with its previously withdrawn BMCA-targeted antibody-drug conjugate, Blenrep.





































Let's personalize your content