Ogsiveo Receives FDA Approval as First Therapy for Desmoid Tumors
XTalks
NOVEMBER 30, 2023
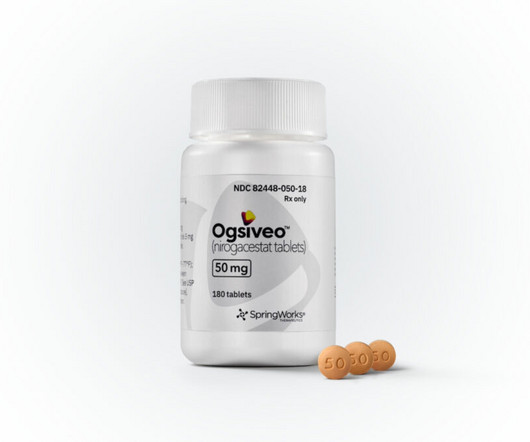
Pfizer spinout SpringWorks Therapeutics’ Ogsiveo (nirogacestat) has received US Food and Drug Administration (FDA) approval for the treatment of desmoid tumors, an ultra-rare subtype of non-cancerous soft tissue sarcomas that can cause severe pain and disfigurement.










































Let's personalize your content