How Potential Changes to the NIH Guidelines Could Impact IBC Review
WCG Clinical
NOVEMBER 17, 2023
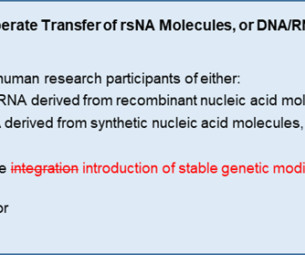
Although the NIH Guidelines were originally written with non-clinical laboratory research in mind, they also apply to human gene transfer (HGT) research, wherein rsNA or rsNA-containing products are administered to research participants. Since then, however, certain genetic engineering technologies (e.g.,
















Let's personalize your content