Touchlight joins forces with University of Liverpool for NSCLC vaccine
Pharmaceutical Technology
APRIL 4, 2024
The vaccines will be developed using Touchlight’s doggybone DNA technology, which speeds up the manufacturing process.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
APRIL 4, 2024
The vaccines will be developed using Touchlight’s doggybone DNA technology, which speeds up the manufacturing process.

Medical Xpress
NOVEMBER 18, 2022
Rutgers scientists have developed a new approach to stopping viral infections: a so-called live-attenuated, replication-defective DNA virus vaccine that uses a compound known as centanamycin to generate an altered virus for vaccine development.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery World
NOVEMBER 20, 2023
Raquel Sanches-Kuiper , Vice President of Science and Applications at Evonetix, and Clare Whitewoods , Marketing Communications Manager at Evonetix, look at the benefits synthetic DNA brings to pharmaceutical development. Their short development timeline gives mRNA vaccines several distinct advantages over traditional vaccines.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. The company provides end-to-end contract development and manufacturing organisation (CDMO) services from the discovery to the commercialisation of drugs.
pharmaphorum
AUGUST 23, 2021
The COVID-19 pandemic accelerated the development of mRNA-based vaccines, and its influence has now extended to DNA-based shots as well, with Zydus Cadila’s ZyCoV-D getting emergency use authorisation in India. Proponents of the approach claim that DNA vaccines may have advantages over other technologies like mRNA.
Bio Pharma Dive
SEPTEMBER 28, 2020
The FDA has suspended the planned start of a late-stage trial of Inovio's experimental shot, dealing another setback to one of the most advanced DNA-based vaccine programs in development.
BioPharma Reporter
AUGUST 2, 2021
With Scancell taking its DNA-based COVID-19 vaccine tech into clinical trials this year, it is already focusing its attention on the techâs potential to be used as a needle-free booster shot to address Variants of Concern.

Drug Discovery World
NOVEMBER 29, 2022
Using artificial intelligence, researchers at Chalmers University of Technology, Sweden have succeeded in designing synthetic DNA that controls the cells’ protein production. . The technology can contribute to the development and production of vaccines and drugs for severe diseases much faster and at lower cost. .

Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections.
BioSpace
MARCH 2, 2021
GE Research selected DNA Script to join a collaboration working on a rapid response, mobile platform to develop on-demand production of nucleic acid-based vaccines and therapies related to biological threats.

BioPharma Reporter
FEBRUARY 17, 2022
Start-up biotech Alvea has started pre-clinical testing for its DNA vaccine: with clinical trials set to begin as soon as March.

Scienmag
APRIL 22, 2021
Dose-sparing regimens and intradermal delivery have important implication for rapid clinical development of effective, well-tolerated and easy-to-distribute vaccines against MERS and other emerging coronaviruses.

Drug Discovery World
OCTOBER 2, 2023
This eBook sponsored by DNA Script explores the advances and breakthroughs taking place within vaccines and virology.
Pharmaceutical Technology
FEBRUARY 15, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Flavivirus vaccine components. Sanofi is one of the leading patent filers for Flavivirus vaccine components.

Pharmaceutical Technology
JUNE 8, 2023
GenScript ProBio has collaborated with the New York Blood Center Enterprises’ business unit, called Comprehensive Cell Solutions (CCS), to accelerate the development and manufacture of cell and gene therapies. The core objective of the collaboration is to make the therapies affordable and accessible to patients.
Pharmaceutical Technology
FEBRUARY 15, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Coronavirus vaccine components. The vaccine also contains other inactive ingredients such as cholesterol.
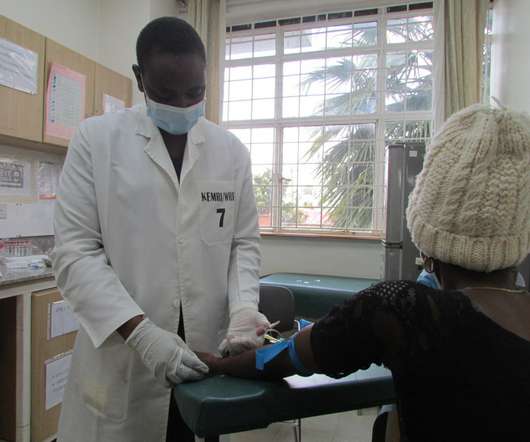
Scienmag
MARCH 15, 2021
The first vaccine has been administered in a comparative adjuvant trial of DNA prime/protein boost HIV vaccine regimens in Kericho, Kenya Credit: U.S. Military HIV Research Program SILVER SPRING, Md.
BioPharma Reporter
FEBRUARY 13, 2023
Scancell has reported preliminary immunogenicity data from its Phase 1 COVIDITY clinical trial: with the plasmid DNA based COVID-19 vaccine candidates inducing immune responses when delivered via needle-free tech.

Drug Discovery World
FEBRUARY 14, 2024
Vaccination responses need to be rapid, efficacious and cost-effective if they are to outpace new viral threats emerging across the globe, says Jian He (Jason) , Chemistry, Manufacturing and Controls (CMC) Head, WuXi Vaccines. This would give more people much faster access to more effective vaccines.

pharmaphorum
MARCH 2, 2021
The COVID-19 pandemic brought life-changing disruptions to people around the world and has turned the typical vaccine R&D process on its head. Overcome immunogenicity challenges and design safe and efficacious vaccine formulations. The post Genetic Vaccine Development for Infectious Diseases Summit appeared first on.

BioTech 365
SEPTEMBER 15, 2020
.–(BUSINESS WIRE)–PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its Stratis® Needle-free Injection System will be used to deliver a novel DNA vaccine against COVID-19 (GX-19) which is being developed by an international consortium led by … Continue reading →

Drug Discovery World
AUGUST 16, 2023
Samir Ounzain , PhD, CEO & Co-Founder of HAYA Therapeutics, looks at how a better understanding of our DNA can lead to increased activity for RNA therapeutics. The whole world realised the power of RNA when the Covid-19 pandemic brought us the first mRNA-based vaccines.

Drug Discovery World
NOVEMBER 23, 2022
One critical bottleneck in Cell and Gene Therapy (CGT) manufacturing is the production of high-quality plasmid DNA (pDNA), a vital building block for mRNA-based vaccines, viral vector-based cell or gene therapies that require pDNA as a starting material.

Drug Discovery World
JUNE 8, 2023
Hosted by DDW and sponsored by DNA Script, ‘Drug discovery and development trends – what will have most impact?’ Join this free roundtable to learn about the biggest opportunities for the future of drug discovery and development. How important will mRNA-based vaccines be in treating viruses and diseases such as cancer?

Drug Discovery World
JUNE 15, 2023
Hosted by DDW and sponsored by DNA Script, ‘Drug discovery and development trends – what will have most impact?’ Join this free roundtable to learn about the biggest opportunities for the future of drug discovery and development. How important will mRNA-based vaccines be in treating viruses and diseases such as cancer?

Drug Discovery World
JUNE 7, 2023
Hosted by DDW and sponsored by DNA Script, join us for this free-to-attend roundtable to learn about the biggest opportunities for the future of drug discovery and development. How important will mRNA-based vaccines be in treating viruses and diseases such as cancer? What can we expect from the cell & gene therapy market?

XTalks
NOVEMBER 6, 2020
Canadian clinical-stage biotech company Symvivo Corporation has developed an oral COVID-19 vaccine that entered clinical trials this week. The first healthy volunteer was dosed with the vaccine in Australia as part of the bacTRL-Spike COVID-19 Phase I clinical trial. Related: Red Meat Allergy Test Gets FDA Clearance. “We
Drug Discovery World
JUNE 21, 2023
Hosted by DDW and sponsored by Elegen, “Where are the breakthroughs in mRNA drug discovery and development?” Join this free webinar to learn about the opportunities in mRNA drug discovery and development. What you will learn: What are the challenges in relation to the development of mRNA therapeutics and how can they be overcome?

The Pharma Data
DECEMBER 23, 2020
” Dr. Stanley Plotkin , Professor Emeritus at The Wistar Institute, said, “INOVIO’s DNA vaccine appeared to be quite safe with few significant reactions but yet induced both antibody and T cell responses to SARS-CoV-2.” . PLYMOUTH MEETING, Pa. , PLYMOUTH MEETING, Pa. ,

XTalks
NOVEMBER 23, 2021
Canadian pharma and biotech contract development and manufacturing organization (CDMO) Biovectra has announced plans for building one of the first state-of-the-art mRNA vaccine and biomanufacturing facilities in Canada. Related: Medicago’s Plant-Based COVID-19 Vaccine Enters Human Trials. million, respectively.

Drug Discovery Today
JULY 28, 2020
Cancer Research UK, the University of Southampton and Touchlight Genetics, a London based biotechnology company, today (Wednesday) announce a new clinical development partnership to progress a therapeutic DNA vaccine, TGL-100, into an early phase clinical trial targeting head and neck squamous cell carcinoma (HNSCC).
pharmaphorum
OCTOBER 2, 2020
Roche’s Genentech unit has paid $200 million to develop an individualised cancer vaccine with Norwegian biotech Vaccibody, which focuses on targets known as neoantigens that spring up as tumours grow and mutate. Frame Therapeutics has also begun work on neoantigen-based cancer vaccines based on RNA technology.
Drug Discovery World
FEBRUARY 28, 2024
Lu Rahman selects some of the year’s interesting and noteworthy advances in cancer research drug discovery and development. As always, a year within cancer drug discovery and development brings with it significant breakthroughs, and 2023 has been no exception. Figures from the Amercian Cancer Society 1 state that “a little over 1.9
BioPharma Reporter
OCTOBER 26, 2023
We took the time to speak with Amy Walker, VP of research, at biotechnology company 4basebio to discuss if synthetic DNA templates represent the future of mRNA production.

Drug Discovery Today
JULY 7, 2022
Pfizer and Touchlight agree to patent license for Pfizer to utilise rapid, scalable, enzymatic doggybone DNA (dbDNA) in Pfizer’s clinical and commercial manufacture of its mRNA vaccines, therapeutics, and gene therapiesAgreement includes upfront payment, potential development and commercial milestone payments, and royalties upon commercializationAccess (..)

pharmaphorum
JANUARY 5, 2023
The company develops mRNA therapeutics and vaccines , and the move will enable it to utilise the synthetic biology and enzyme tech of 2018-founded Japanese company OriCiro, which develops cell-free DNA synthesis and amplification technologies, and thereby expand Moderna’s portfolio. Biotech Moderna, Inc.

The Pharma Data
NOVEMBER 26, 2020
26, 2020 /PRNewswire/ — BASE10 Genetics and DNA Link today announced their collaboration on a research project to evaluate the usability of DNA Link ‘ s AccuFind COVID-19 IgG antibody test in a healthcare setting. “ DNA Link’s AccuFind performs very well in laboratory settings. About DNA Link, Inc.
pharmaphorum
OCTOBER 12, 2022
Now, he leads Whitelab Genomics as its artificial intelligence (AI) platform powers the development of genomic therapies – an emerging field in which genetic sequences are injected into cells to target and repair damaged genes. The payload, Del Bourgo tell us, is the therapeutic DNA or RNA sequence that will cure or fix damaged cells.

STAT News
OCTOBER 5, 2022
Scientists rely on gene synthesis technologies as a research tool for everything from basic research to vaccine development and drug target identification. Ever since the inception of gene synthesis, there have been concerns about possible misuse of synthetic genes.

pharmaphorum
SEPTEMBER 29, 2020
Inovio Pharma’s plan to start pivotal testing of its COVID-19 vaccine has been delayed by FDA requests for more information, sending its shares into a swift decline. Inovio’s vaccine takes the form of a DNA plasmid coding for the full length of the spike or ‘s’ glycoprotein antigen of SARS-CoV-2, the vaccine that causes COVID-19.
The Pharma Data
DECEMBER 20, 2020
UK drug developer Scancell said it has chosen a COVID-19 vaccine candidate, SN14, from more than a dozen potential products to advance to a clinical trial. . SN14 works by targeting the coronavirus’ nucleocapsid and spike proteins to prevent viral replication using the company’s ImmunoBody DNA vaccine technology.

Scienmag
JUNE 16, 2022
To develop new drugs and vaccines, detailed knowledge about nature’s smallest biological building blocks – the biomolecules – is required.

Drug Discovery World
JULY 22, 2022
Covid-19 vaccines remain effective six months after second dose . Two doses of Covid-19 vaccines were sufficient in providing protection against severe cases of the infectious disease, according to new research.? . Junk DNA could lead to cancer, study says . News round-up by DDW’s Reece Armstrong for 18 – 22 July.

Drug Discovery World
MARCH 14, 2023
The success of messenger RNA or mRNA vaccines in the fight against the global SARS-CoV-2 crisis brings a new era of vaccine development. In vitro transcription, or IVT, is a complex reaction, requiring key components like an RNA polymerase, nucleotides, and a DNA template to make each mRNA.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content