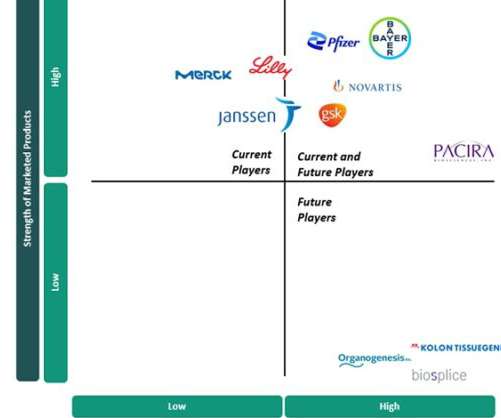
Current and future players in the osteoarthritis market
Pharmaceutical Technology
JULY 28, 2022
The current standards of care (SOCs) in OA focus on symptom management and are made up of generic pharmaceuticals, including nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, antidepressants and intra-articular (IA) injections. Eli Lilly is another key player in the OA market due to duloxetine.
































Let's personalize your content