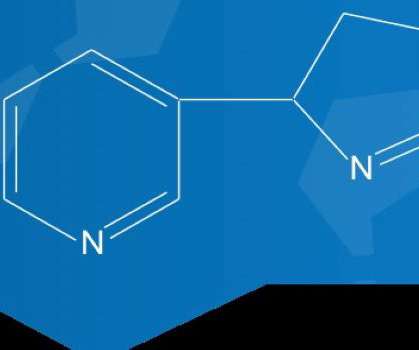
Apoptosis Regulator BAX drugs in development, 2023
Pharmaceutical Technology
DECEMBER 7, 2023
GlobalData’s report assesses the drugs in the Apoptosis Regulator BAX pipeline by therapy areas, indications, stages, MoA, RoA, molecule type and the key players in the development pipeline.








































Let's personalize your content