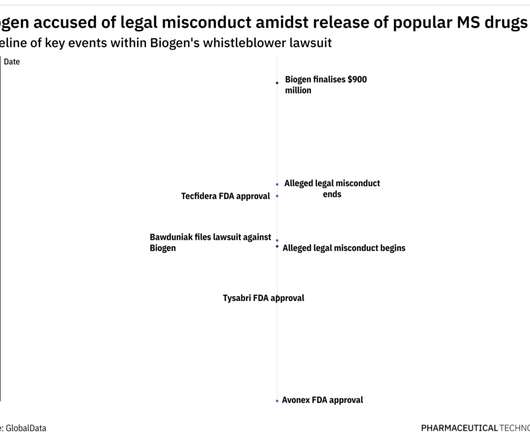
FDA approves Coherus’ Cimerli for DME, with year-long interchangeability exclusivity
Pharmaceutical Technology
AUGUST 5, 2022
In addition, for doctors who do use Lucentis, there will be added pressure from payers to switch to Cimerli with its direct interchangeability with Lucentis approved. The post FDA approves Coherus’ Cimerli for DME, with year-long interchangeability exclusivity appeared first on Pharmaceutical Technology.











































Let's personalize your content