Konvomep Gets FDA Approval as a New Oral Liquid Formulation Option of Omeprazole
XTalks
SEPTEMBER 6, 2022
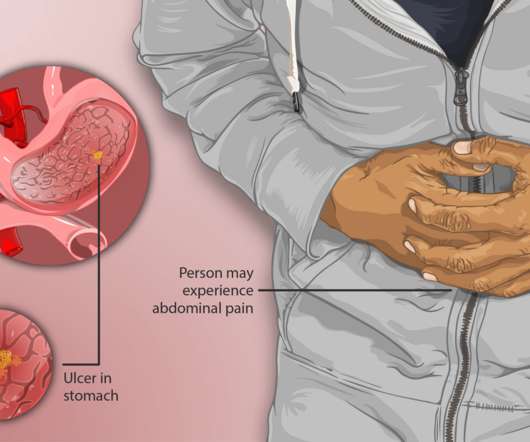
Gastric ulcers may also be caused by an overuse of NSAIDs like ibuprofen or aspirin, especially if these anti-inflammatory medicines were taken at high doses or for a long time. Patients are our priority, and our purpose is to bring them new formulations that help them benefit from established medicines.







































Let's personalize your content