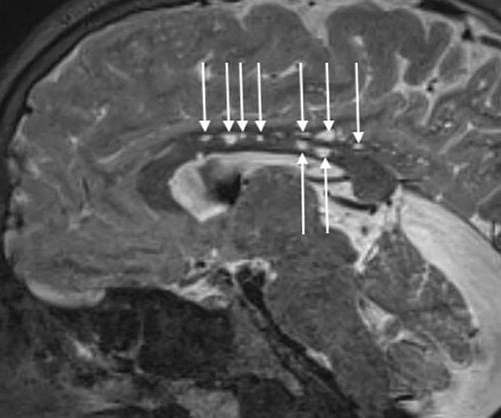
FDA clears Intellia to start US tests of ‘in vivo’ gene editing drug
Bio Pharma Dive
MARCH 2, 2023
The announcement, which follows recent regulatory setbacks for some of Intellia’s peers, is a “big win” for the gene editing field, according to one analyst.







































Let's personalize your content