Single-Arm Trials Using Real-World Evidence for Rare Disease Product Development
Camargo
OCTOBER 15, 2020
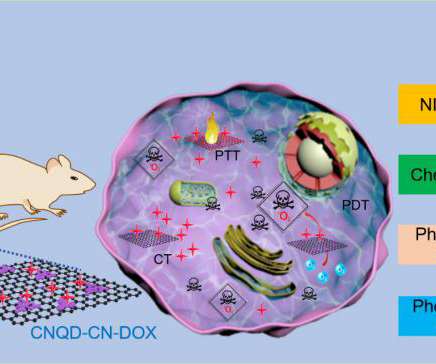
As a sponsor designs clinical studies, the respective comparison control groups become a critical factor to consider. Often, to gain clinical trial design insights, a sponsor reviews the physician package inserts from approved New Drug Applications (NDAs) and Biologics License Applications (BLAs) with similar indications or in the same therapeutic area.


































Let's personalize your content