iNtRON wins US Army contract to develop bacteriophages for E.coli infections
Pharmaceutical Technology
SEPTEMBER 4, 2023
iNtRON's contract is part of the US Army’s DEVCOM strategy to develop drugs to treat antibiotic-resistant UTIs.

Pharmaceutical Technology
SEPTEMBER 4, 2023
iNtRON's contract is part of the US Army’s DEVCOM strategy to develop drugs to treat antibiotic-resistant UTIs.
Outsourcing Pharma
SEPTEMBER 4, 2023
The UK startup Intelligent OMICS (Intellomx) has launched a collaboration with Johnson & Johnsonâs pharmaceutical company Janssen to use artificial intelligence (AI) to discover drug targets for the treatment of blood cancer.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
SEPTEMBER 4, 2023
The Japanese MHLW has accepted GSK’s supplementary new drug application (sJNDA) for Nucala (mepolizumab) to treat chronic rhinosinusitis
BioPharma Reporter
SEPTEMBER 4, 2023
Quest Diagnostics, a provider of diagnostic information services, has announced that its AAVrh74 ELISA assay (CDx) has been granted breakthrough device designation from the U.S. Food and Drug Administration (FDA).

Pharmaceutical Technology
SEPTEMBER 4, 2023
The company published a study showing lyso-Gb1 (glucosylsphingosine) as a diagnostic and predictive biomarker for the rare metabolic disease.

Outsourcing Pharma
SEPTEMBER 4, 2023
Less than a month after the U.S. Food and Drug Administration (FDA) gave its antidepressant drug zuranolone (Zurzuvae) a mixed welcome, Sage Therapeutics has launched plans to reorganize its operations by refocusing its drug development efforts and laying off 40% of its workforce.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Drug Patent Watch
SEPTEMBER 4, 2023
Annual Drug Patent Expirations for SPINRAZA Spinraza is a drug marketed by Biogen Idec and is included in one NDA. It is available from one supplier. There are nine patents… The post New patent expiration for Biogen Idec drug SPINRAZA appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
SEPTEMBER 4, 2023
Biocon Generics has purchased the US-based oral solid dosage manufacturing facility of Eywa Pharma for a total consideration of $7.7m.
Outsourcing Pharma
SEPTEMBER 4, 2023
The small molecule drug alectinib (Alecensa) developed by the big pharma company Roche has improved disease-free survival in patients with a specific form of non-small cell lung cancer (NSCLC) when delivered as an adjuvant therapy in a phase 3 trial.

Pharmaceutical Technology
SEPTEMBER 4, 2023
Amgen and Horizon Therapeutics have reached a consent order agreement with the FTC to resolve the ongoing administrative lawsuit.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Drug Patent Watch
SEPTEMBER 4, 2023
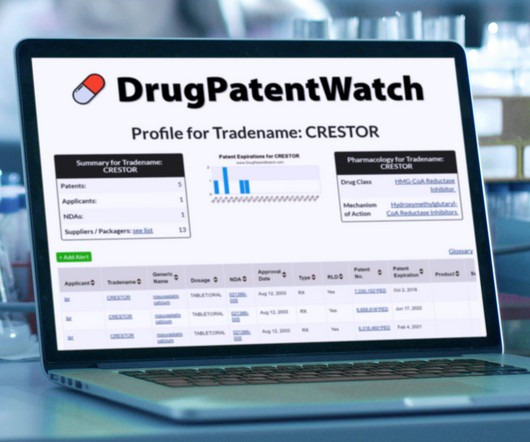
Annual Drug Patent Expirations for KYNAMRO Kynamro is a drug marketed by Kastle Theraps Llc and is included in one NDA. There are three patents protecting this drug. This drug… The post New patent expiration for Kastle Theraps drug KYNAMRO appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
SEPTEMBER 4, 2023
Private Equity firm Permira has announced that it is taking the company private, following many other UK firms.

Pharma Times
SEPTEMBER 4, 2023
The therapy has been developed for the treatment of Fabry disease among adult patients - News - PharmaTimes
BioPharma Reporter
SEPTEMBER 4, 2023
With the rise of mRNA-based treatments, the field of oncology is about to go through a significant change.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharma Times
SEPTEMBER 4, 2023
The therapy has been developed for the treatment of migraine among adults - News - PharmaTimes

pharmaphorum
SEPTEMBER 4, 2023
Patient involvement at NICE: In whose interest is it anyway? Mike.

Drug Patent Watch
SEPTEMBER 4, 2023
Annual Drug Patent Expirations for QUTENZA Qutenza is a drug marketed by Averitas and is included in one NDA. It is available from one supplier. There are seven patents protecting… The post New patent expiration for Averitas drug QUTENZA appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
SEPTEMBER 4, 2023
Ergomed aims to go private via £703m Permira takeover Phil.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
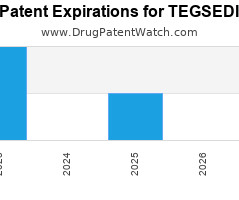
Drug Patent Watch
SEPTEMBER 4, 2023
Annual Drug Patent Expirations for TEGSEDI Tegsedi is a drug marketed by Akcea Theraps and is included in one NDA. It is available from one supplier. There are six patents… The post New patent expiration for Akcea Theraps drug TEGSEDI appeared first on DrugPatentWatch - Make Better Decisions.

Drug Discovery World
SEPTEMBER 4, 2023
Kadans’ London Innovation Centre at Canary Wharf, London has launched its wet lab innovation and flexible workspace centre and welcomed a new tenant. AviadoBio, a gene therapy company developing medicines for neurodegenerative disorders, will establish laboratory and office presence at the Innovation Centre. The Innovation Centre has enabled the rapid growth of the life sciences hub at Canary Wharf , providing opportunities for early-stage life science companies to collaborate and innovate.

pharmaphorum
SEPTEMBER 4, 2023
Wegovy finally reaches the UK, in 'limited' quantities Phil.

Drug Discovery World
SEPTEMBER 4, 2023
In the third and final part of this series of articles focused on plant-based medicines, DDW’s Megan Thomas evaluates therapeutic breakthroughs using plant-based products in drug discovery. The first part of the series explored medical cannabis , and the second looked into psylocibin. Green tea Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is a type of catechin that is found in green tea.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
pharmaphorum
SEPTEMBER 4, 2023
Roche seeks early-stage lung cancer use for Alecensa Phil.

Trialfacts
SEPTEMBER 4, 2023
Contents About the Study Why Participate? Your Rights Who Can Participate? More Study Details About the Research Center: Study Location Research Center: Indiana University Location: 362 W 15th St, Indianapolis, IN 46202 Lead Researcher: Dr. Susan Conroy and Dr. Steve Strakowski (Co-PI) IRB: This study has been reviewed and approved by the Local IRB at Indiana University About the Study Did you know that bipolar disorder affects a whopping 45 million people worldwide and about 2.8% of US adults p

Outsourcing Pharma
SEPTEMBER 4, 2023
Andrew MacGarvey is chief operating officer (COO) of Phastar. We caught up with him earlier in the summer at DIA Global in Boston to discuss the companyâs origins, opportunities for big analytical data DCTs, artificial intelligence and machine learning among many other topics.

Drug Discovery World
SEPTEMBER 4, 2023
SLAS2024 event partner DDW will be the exclusive sponsor for the SLAS Ignite Theater, hosting its own free to attend seminar series, ‘Innovation & technology: from lab to patient’. Join DDW at SLAS2024 in Boston on 5 and 6 February 2024 to explore how drug discovery technology is driving innovation from bench to bedside. Inspired by the event theme of ‘Innovation at Every Turn’, the DDW track will present leading research and case studies from industry experts in the pharmaceutical, biopharm

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Roots Analysis
SEPTEMBER 4, 2023
In the recent years, service industry has become a fast-growing segment in the overall healthcare domain – thanks to the steady increase in demand for contract research organizations (CROs) and contract manufacturing organizations. The need to focus on developing more efficient interventions, within stipulated timelines and budget constrains is the key driver for outsourcing operations to third-parties and the diagnostics development space is no exception.

Drug Discovery World
SEPTEMBER 4, 2023
The latest episode of the DDW Highlights podcast is now available to listen to below. DDW’s Megan Thomas narrates five key stories of the week to keep DDW subscribers up-to-date on the latest industry updates. This week’s headline news features a stem-cell derived Parkinson’s therapy, a monoclonal antibody for head and neck cancer, an estrogen receptor degrader for breast cancer, a mitochondrial activator for long Covid, and the world’s first AI-designed bispecific T cell engager – reflecting
BioPharma Reporter
SEPTEMBER 4, 2023
Optibrium, a developer of software and AI solutions for drug discovery, has acquired BioPharmics in a view to expand its 3D drug design and modelling offering.

Drug Discovery World
SEPTEMBER 4, 2023
The latest episode of the DDW Highlights podcast is now available to listen to below. DDW’s Megan Thomas narrates five key stories of the week to keep DDW subscribers up-to-date on the latest industry updates. This week’s headline news features a stem-cell derived Parkinson’s therapy, a monoclonal antibody for head and neck cancer, an estrogen receptor degrader for breast cancer, a mitochondrial activator for long Covid, and the world’s first AI-designed bispecific T cell engager – reflecting

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content