Ginkgo grows its gene therapy offerings with StrideBio deal
Bio Pharma Dive
APRIL 5, 2023
The deal hands Ginkgo technology for discovering and engineering capsids — the outer shell that protects the helpful genetic material in gene therapies.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Bio Pharma Dive
APRIL 5, 2023
The deal hands Ginkgo technology for discovering and engineering capsids — the outer shell that protects the helpful genetic material in gene therapies.
Pharmaceutical Technology
JANUARY 5, 2023
Capsida Biotherapeutics and Eli Lilly and Company ’s wholly owned subsidiary Prevail Therapeutics have announced a partnership for the development of non-invasive gene therapies for central nervous system (CNS) diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 25, 2023
ElevateBio has raised $401m in a Series D financing round for advancing its technology platforms to expedite the design, production and development of cell and gene therapies. ElevateBio intends to use the funds to advance its genetic medicine current good manufacturing practice (cGMP) and process development business, BaseCamp.

Pharmaceutical Technology
APRIL 6, 2023
Ginkgo Bioworks has strengthened its end-to-end R&D capabilities in gene therapy with the purchase of adeno-associated virus (AAV) capsid discovery and engineering assets from StrideBio, for an undisclosed sum. The new capabilities and IP will be incorporated under Ginkgo’s end-to-end AAV gene therapy development platform.

Velocity Clinical Research
OCTOBER 3, 2024
As Nick Spittal states in this Advarra press release, membership in the Gene Therapy Ready (GTR) site network “allows Velocity to start studies over a month faster and provides a meaningful credential and important validation that increases sponsors’ confidence in our specialized capabilities to conduct complex clinical research safely.”

Pharmaceutical Technology
AUGUST 2, 2022
Samsung Biologics and GreenLight Biosciences have completed the initial commercial-scale engineering run for their messenger ribonucleic acid (mRNA) Covid-19 vaccine under their manufacturing collaboration. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Pharmaceutical Technology
DECEMBER 14, 2022
Merck and Synplogen have signed a non-binding Memorandum of Understanding (MoU) to expedite the development and manufacturing of viral vector-based gene therapy applications. The firms intend to merge their expertise to provide simplified viral vector gene therapy development, production and testing in Japan.

BioPharma Reporter
SEPTEMBER 5, 2024
In one of the largest cell and gene therapy funding rounds this year, the US company Arsenal Biosciences has secured $325 million in a Series C round to bankroll T cell therapies engineered to take on solid tumors.

Cloudbyz
JULY 31, 2024
This revolution is enabling the growth of innovative biomarker-based precision medicine and cell and gene therapy, transforming both clinical research and post-market care. These therapies involve modifying a patient’s cells or genes to treat or prevent diseases, with the promise of long-lasting effects.

BioTech 365
JANUARY 19, 2022
64x Bio Raises $55 Million Series A Financing to Advance Cell Line Engineering Platform for Gene Therapy Manufacturing 64x Bio Raises $55 Million Series A Financing to Advance Cell Line Engineering Platform for Gene Therapy Manufacturing Multibillion-dollar gene therapy market … Continue reading →
Pharmaceutical Technology
NOVEMBER 16, 2022
ElevateBio has entered a partnership with Affini-T Therapeutics to progress the latter’s engineered TCR-T therapies focused on Kirsten rat sarcoma viral oncogene homolog (KRAS), a dominant oncogenic driver mutation in solid tumours. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
pharmaphorum
JUNE 15, 2021
When Biogen acquired Nightstar Therapeutics in 2019, it added a pair inherited retinal disorder (IRD) gene therapies that it hoped would accelerate a push into the fast-evolving category. . The post Biogen’s ambitions in gene therapy hit by another failed pivotal trial appeared first on.

BioTech 365
OCTOBER 27, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: ElevateBio cuts a deal for Life Edit, adding genome engineering tech to support gene therapy pipeline.ElevateBio cuts a deal for Life Edit, adding genome engineering tech to … Continue reading →

pharmaphorum
OCTOBER 22, 2020
Sarepta chief commercial officer Bo Cumbo has left to head up gene therapy venture – AavantiBio – with $107 million in backing from his former employer and three high-profile life sciences investors. Unlike a one-shot gene therapy, omaveloxolone would require continuous dosing to maintain its effects.
Roots Analysis
NOVEMBER 30, 2021
Bioassay – A Prerequisite for the Cell and Gene Therapy Development. Over the past few years, investigational new drug (IND) filings for cell and gene therapy product have significantly increased. Companies Offering Bioassay Services for Cell and Gene Therapies.

Advarra
NOVEMBER 29, 2022
The field of cell and gene therapies (CGT) is constantly evolving, and there has been significant progress in this area of research. However, despite the promise of these therapies, the regulations governing them lag the science, which in turn hinders the clinical translation of these novel medicines.

BioPharma Reporter
APRIL 11, 2023
Ginkgo acquires StrideBio's adeno-associated virus (AAV) capsid discovery and engineering platform assets and as well as gaining ownership of a preclinical asset.

BioSpace
MAY 10, 2021
Biogen and Capsigen forged a strategic collaboration to engineer novel adeno-associated virus (AAV) capsids that have the potential to become transformative gene therapies that treat underlying genetic causes of various central nervous system and neuromuscular disorders.

Scienmag
JUNE 9, 2021
Credit: RCSI Scientists have developed polypeptide-based materials that act as effective vectors for delivering gene therapies. The first-of-its-kind platform enables the vectors to be adapted to suit the specific gene therapy cargo.
STAT News
AUGUST 18, 2022
The therapy, Zynteglo, is just the third gene therapy approved by the FDA, and the first to target a chronic blood disease. It uses an engineered virus to deliver a gene fix into the bone marrow of patients. Zynteglo will cost $2.8

The Pharma Data
JANUARY 4, 2021
Collaboration with Biogen to develop gene therapy for an undisclosed target to treat inherited eye disease, plus option for additional target. 05, 2021 (GLOBE NEWSWIRE) — ViGeneron GmbH , a gene therapy company, today announced a global collaboration and licensing agreement with Biogen Inc.
STAT News
JULY 11, 2022
Twenty-three years ago, the field of gene therapy was bursting with the promise of breakthrough treatments. Then it was almost instantly derailed by the death of an 18-year-old clinical trial volunteer named Jesse Gelsinger after he received a genetically engineered virus that had been developed to treat his rare liver condition.

The Pharma Data
JANUARY 25, 2021
a biotechnology company developing cell and gene therapy treatments for patients suffering from vascular disease, today announced that it raised up to $5.5M that will lead clinical testing of a novel gene therapy for a serious vascular disease in Europe. MIAMI, Jan. 25, 2021 (GLOBE NEWSWIRE) — Ambulero, Inc. ,

Advarra
MARCH 31, 2023
The use of engineered genetic materials in clinical trials is rapidly expanding, with potential applications for genetic vaccines, gene-modified cellular therapies, and gene therapies. A key part of the IBC’s evaluation is assessing the risks posed by the engineered genetic materials.

Advarra
JUNE 13, 2024
Genetically engineered products often require additional safety practices to ensure the infectious agents do not endanger participants, study staff, or the broader environment where such agents are administered. The post The Importance of Hazard Communications in Clinical Trials Involving Genetic Engineering appeared first on Advarra.

BioSpace
FEBRUARY 2, 2021
The next several years will be transformative in the cell and gene therapy space, and Immatics’ Chief Medical Officer Cedrik M. Britten believes his company’s engineered T-cell receptors may play an important role in providing treatment options for cancer patients.
Pharmaceutical Technology
APRIL 20, 2023
Circio aims to develop new circRNA medicines initially for cancer, then plans to expand rapidly into vaccines and gene therapy. CircAde is said to be the most advanced therapeutic concept, and leverages the company’s experience in oncolytic viruses to use engineered adenoviruses to deliver circRNA to cancer cells.
The Pharma Data
AUGUST 24, 2020
The FDA has approved a request from American Gene Technologies to begin a clinical study into its HIV gene therapy. This requires an 11-day programme which involves extracting blood from an HIV patient and separating their T cells, which are then engineered in a lab to make them immune to HIV.
Pharmaceutical Technology
APRIL 14, 2023
The company can use its mammalian cell engineering expertise and capabilities to screen CAR T-cells, to discover and optimise next-generation therapeutic candidates for its partners. WARF and Ginkgo Bioworks also intend to partner on developing a pooled in vivo screening platform to further advance the discovery of new CAR therapies.

The Pharma Data
OCTOBER 18, 2020
In late August, Pfizer announced it was investing an additional $500 million into its state-of-the-art gene therapy manufacturing facility in Sanford, North Carolina. Ricci told BioSpace the company has been “investing heavily in gene therapy and in the Raleigh-Durham Research Triangle Park area.”
Pharmaceutical Technology
APRIL 28, 2023
The US Food and Drug Administration (FDA) has granted an Orphan Drug Designation to Editas Medicine’s gene therapy EDIT-301 in sickle cell disease, based on an April 27 announcement. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. This increases the expression of fetal haemoglobin.

Pharmaceutical Technology
JUNE 12, 2023
Quell Therapeutics and AstraZeneca have entered a collaboration, exclusive option and licence deal for the development, manufacturing and commercialisation of engineered T-regulator (Treg) cell therapies for autoimmune diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Scienmag
OCTOBER 19, 2020
New generation CRISPR technology lays foundation for therapeutics to treat a wide range of inherited ocular diseases Credit: Nature Biomedical Engineering Irvine, CA – October 19, 2020 – A breakthrough study, led by researchers from the University of California, Irvine, results in the restoration of retinal and visual functions of mice (..)

Pharmaceutical Technology
AUGUST 26, 2022
ElevateBio has signed a long-term strategic collaboration with the University of Pittsburgh, US, to establish a biomanufacturing centre for expediting cell and gene therapy development. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

The Pharma Data
JANUARY 17, 2021
The companies will evaluate ViGeneron’s proprietary, intravitreally injected vgAAV vectors for delivering a novel therapeutic protein to develop a gene therapy treatment for a highly prevalent eye disease. There is significant unmet medical need for a sustained therapy to treat eye diseases. MUNICH, Germany, Jan.

Pharmaceutical Technology
OCTOBER 13, 2022
Inceptor Bio has partnered with cell engineering technology company Avectas to improve CAR-T cell therapies’ development and manufacturing to treat solid tumours. Under the collaboration deal, Inceptor Bio will use the Solupore technology from Avectas instead of electroporation for engineering T cells to yield a healthier T cell.

Pharmaceutical Technology
MAY 3, 2023
BrightPath Biotherapeutics CEO Kenichi Nagai stated: “This collaboration with Artisan provides us with the potential to create highly engineered allogeneic iNKT cellular therapy programmes for a range of indications, including solid tumours. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Pharmaceutical Technology
JANUARY 4, 2023
Cell-engineering company MaxCyte has signed a strategic platform licence (SPL) with biotechnology company Catamaran Bio to support the chimeric antigen receptor (CAR)-NK cell therapy programmes. These CAR-NK cell therapies combine new functional attributes with the NK cells’ inherent cancer-fighting properties.
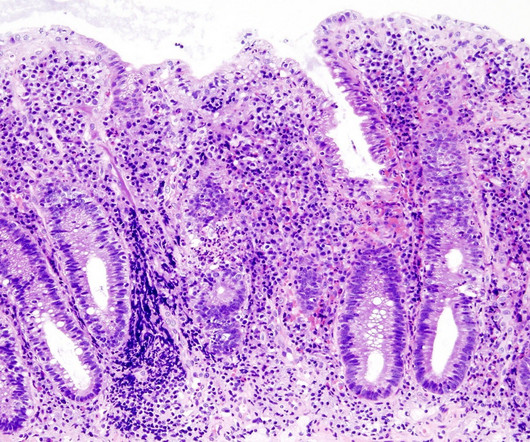
Pharmaceutical Technology
MARCH 29, 2023
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. Also included are reviews of key studies from scientific literature and a consideration of the interactions between cooling and warming rates, as applicable to cell and gene therapies.

Scienmag
MARCH 1, 2021
The in-person Cell Culture Engineering XVII Conference has been postponed until October 16 – October 22, 2021 in Tucson, Arizona. To highlight the key role that the CCE community plays to free people worldwide from the shackles of the COVID-19 pandemic, we are planning two webinars during the original dates of the conference.
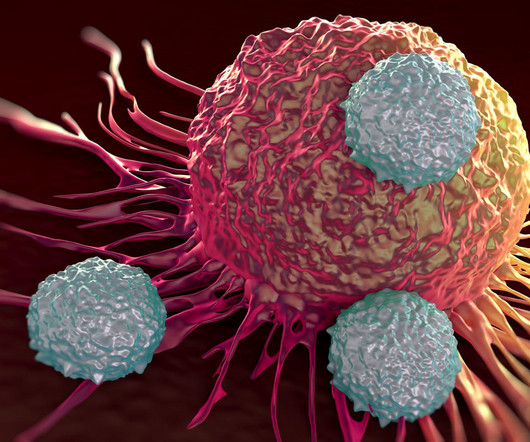
Pharmaceutical Technology
MAY 31, 2023
The company is also looking at developing therapies based on individual patient neoantigens in patient human leukocyte antigens (HLAs). Anocca’s platform features libraries of engineered human cells that allow for standardised analysis of T-cell biology, per the company’s website.
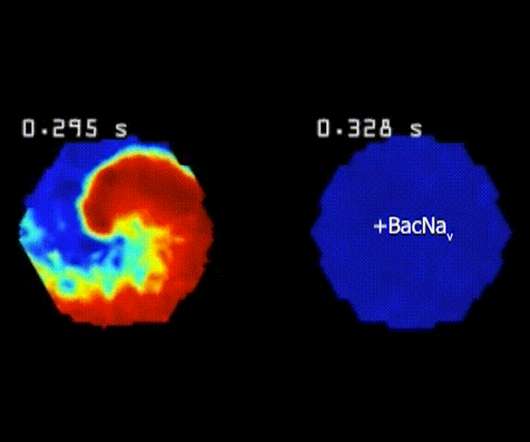
Scienmag
FEBRUARY 4, 2022
Biomedical engineers at Duke University have demonstrated a gene therapy that helps heart muscle cells electrically activate in live mice. DURHAM, N.C.

Pharmaceutical Technology
APRIL 5, 2023
But while companies continue studying allogeneic CAR-T therapies, including for their coveted use in solid tumours, such advancements remain challenging. More broadly however, several advancements are on the horizon for cell and gene therapies in 2023. AZ: Cell and gene therapies often come with a high price.
Pharmaceutical Technology
AUGUST 11, 2022
GentiBio and Bristol Myers Squibb (BMS) have signed a multi-year partnership agreement for developing new engineered Treg therapies for inflammatory bowel disease (IBD) patients. These therapies will be developed for re-instating immune tolerance and repairing tissue in IBD.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content