Meitheal partners with Chinese company for US licencing of insulin biosimilars
Pharmaceutical Technology
SEPTEMBER 21, 2023
The licence grants Meitheal marketing rights for insulin aspart, lispro, and glargine following FDA approval.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
SEPTEMBER 21, 2023
The licence grants Meitheal marketing rights for insulin aspart, lispro, and glargine following FDA approval.

Bio Pharma Dive
NOVEMBER 18, 2022
Eli Lilly’s long-acting copycat drug, first approved in late 2021, now has a designation that will allow pharmacists to swap it for Sanofi’s Lantus.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 12, 2022
Novo Nordisk has recently completed its six-part ONWARDS Phase III trial, as ONWARDS 5 reached its primary endpoint with Icodec demonstrating non-inferiority in reducing hemoglobin A1C (HbA1c) in patients with type 2 diabetes (T2D) at week 52 in comparison to once-daily basal insulin analogs. Patients had an overall baseline HbA1c of 8.9%
BioPharma Reporter
MAY 5, 2022
Oramed Pharmaceuticals announced this week that it has enrolled 100% of the patients in the worldâs first Phase 3 study of oral insulin under FDA approved protocols.

Bio Pharma Dive
JULY 29, 2021
An injectable insulin from Viatris has become the first-ever biosimilar product that can be directly substituted for a marketed biologic, a long-awaited decision that could put pricing pressure on other diabetes drugs.

XTalks
AUGUST 30, 2023
million insulin pricing settlement. The states join six others who have been fighting Eli Lilly in court since 2017, claiming that the company hiked prices of its insulin product Humalog. Hear more about the insulin pricing controversy in this episode. The states say the $13.5 The states say the $13.5

BioSpace
JUNE 28, 2023
Of the 30 patients given CellTrans’ Lantidra in two studies, 21 were insulin-free for at least a year and 10 were insulin-free for more than five years.
The Pharma Data
JULY 29, 2021
Food and Drug Administration approved the first interchangeable biosimilar insulin product, indicated to improve glycemic control in adults and pediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research.
pharmaphorum
MAY 16, 2022
It was also compared to a placebo, a GLP-1 receptor agonist (semaglutide) and two long-acting insulin analogues. more than placebo when used in combination with a long-acting insulin. more than insulin degludec and 1.0% more than insulin glargine. more than insulin degludec and 1.0% more than insulin glargine.

pharmaphorum
JULY 29, 2021
Generic drugmaker Mylan has become the first company to secure FDA approval for a biosimilar product that is considered completely interchangeable with the reference product – namely Sanofi’s once-daily insulin Lantus.

XTalks
SEPTEMBER 14, 2022
In this episode, Ayesha discussed the FDA approval of Sanofi’s enzyme replacement therapy Xenpozyme for the treatment of non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD), a rare genetic lysosomal storage disease, in adults and pediatric patients.
Pharmaceutical Technology
APRIL 17, 2023
The US Food and Drug Administration (FDA) has approved an update to the indications and usage section of Horizon Therapeutics ’ Tepezza (teprotumumab-trbw) label to specify its use to treat thyroid eye disease (TED) patients regardless of disease activity or duration.

XTalks
OCTOBER 22, 2021
In July 2021, the agency approved the first interchangeable biosimilar product, Semglee (insulin glargine-yfgn) for the treatment of diabetes. Semglee is both biosimilar to, and interchangeable with, Lantus (insulin glargine). Biosimilars are sometimes mistakenly likened to generics, but the two are not the same.

XTalks
FEBRUARY 8, 2024
Ozempic (Semaglutide) Ozempic sales in 2022: $8.713 billion Company/Developer: Novo Nordisk Date of first FDA approval: December 5, 2017 Indications Ozempic is FDA-approved for: Type 2 diabetes Price of Ozempic: $1,029 for 1.5 Read on to learn more about the top 15 diabetes drugs in 2023, based on 2022 sales statistics.
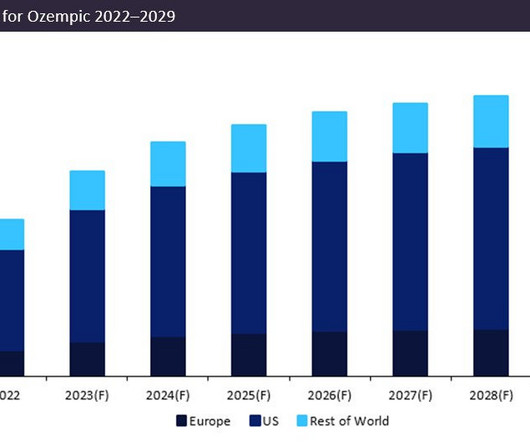
Pharmaceutical Technology
APRIL 28, 2023
Originally granted approval in its largest market, the US, in 2017, Ozempic has subsequently obtained approval for three distinct dosages, 0.5mg, 1.0mg, and 2.0mg, for the treatment of type 2 diabetes. This represents a significant 83% increase in sales between 2022–2029, demonstrating a compound annual growth rate (CAGR) of 9%.
XTalks
JANUARY 25, 2023
Massachusetts-based TheracosBio has received US Food and Drug Administration (FDA) approval for diabetes med Brenzavvy (bexagliflozin) to help improve glycemic control in adults with type 2 diabetes. It is the first FDA-approved SGLT2 inhibitor for any animal species.

Drug Discovery World
FEBRUARY 23, 2024
In a breakthrough for advanced therapies, this week saw the FDA approve the first ever cell therapy for solid tumour cancers, but there were other significant developments in the cell and gene therapy space.
Pharmaceutical Technology
MARCH 1, 2023
More than a century after an individual with type 1 diabetes was treated with insulin for the first time, the FDA approved Tzield, the first and only treatment to delay the onset of type 1 diabetes. Also in this month’s issue, we take a look at the innovative type 1 diabetes therapy by Provention Bio called Tzield.

XTalks
NOVEMBER 22, 2022
Provention Bio’s Tzield (teplizumab) has won US Food and Drug Administration (FDA) approval to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients eight years of age and older who have stage 2 type 1 diabetes. In the US, it is estimated that 1.45 million people are currently living with type 1 diabetes.

XTalks
NOVEMBER 23, 2022
In this episode, Ayesha talked about the breakthrough FDA approval of Provention Bio’s Tzield for delaying the onset of type 1 diabetes in adult and pediatric patients. It’s the first approved treatment for slowing the progression of stage 2 type 1 diabetes to stage 3, the stage at which a clinical diagnosis is made.

FDA Law Blog
AUGUST 12, 2021
Koblitz — About two weeks ago, FDA made an exciting announcement (and it remains exciting even if we’re late posting about it): FDA approved the first interchangeable biosimilar. So, it remains to be seen if and when interchangeable Semglee will have any meaningful effect on insulin prices in the near future.

Drug Discovery World
JULY 10, 2023
In other headline news, the FDA approved the first cellular therapy for type 1 diabetes which could allow some patients to become insulin-independent, and a new study linked severe Covid-19 outcomes with high levels of antibiotics use.

Drug Discovery World
JULY 10, 2023
In other headline news, the FDA approved the first cellular therapy for type 1 diabetes which could allow some patients to become insulin-independent, and a new study linked severe Covid-19 outcomes with high levels of antibiotics use.

XTalks
SEPTEMBER 3, 2020
A leader in the medical device sphere, Medtronic plc announced the release of a US Food and Drug Administration (FDA) approved device called the MiniMed 770G hybrid closed loop system. This is an insulin pump system that offers the company’s most advanced SmartGuard technology. There are 1.25

Drug Discovery World
JULY 7, 2023
In other headline news, the FDA approved the first cellular therapy for type 1 diabetes which could allow some patients to become insulin-independent, and a new study linked severe Covid-19 outcomes with high levels of antibiotics use.
pharmaphorum
NOVEMBER 18, 2022
At its second attempt, Provention Bio has secured FDA approval for teplizumab, as a treatment to delay late-stage type 1 diabetes (T1D) in at-risk individuals – becoming the first disease-modifying therapy for these patients. It is now in line for a $35 million equity investment by Sanofi following FDA approval.

XTalks
JUNE 5, 2023
GLP-1 stimulates insulin production, thus reducing blood glucose levels, and it interacts with the brain to suppress appetite and create a feeling of fullness. As of May 2023, both Ozempic and Wegovy were on the FDA’s Drug Shortages list due to a global shortage of semaglutide.

pharmaphorum
JANUARY 28, 2021
One of these markets is the insulin market. Up until now, the insulin market has experienced little competition and, as a result, prices have soared over the years. Currently, more than seven million patients rely on insulin, and that number is growing each year. But, that will soon be changing, due to biosimilars.
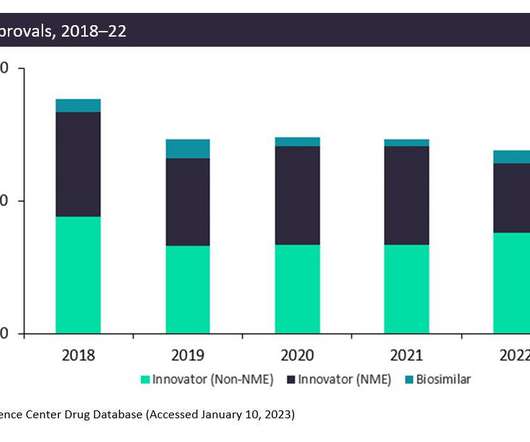
Pharmaceutical Technology
JANUARY 30, 2023
The FDA approved fewer innovative drugs, New Molecular Entities (NMEs), in 2022 than it did in 2021: only 42 drugs compared to 59 drugs. This is due to generally more stringent criteria on approvals in the wake of the Aduhelm scandal. However, non-NME and biosimilar approvals increased in 2022.
STAT News
NOVEMBER 18, 2022
Diabetic patients will now have easier access to insulin after the FDA approved an Eli Lilly biosimilar as interchangeable with the biologic drug , Bloomberg News notes. Rezvoglar, As of 2019, about 1.9 million people have type 1 diabetes in the U.S., Type 1 affects 8% of everyone with diabetes.
The Pharma Data
AUGUST 17, 2021
approval of pump use for Lilly’s novel insulin is latest development designed to help people with diabetes manage blood sugar levels. Lyumjev, a novel formulation of insulin lispro developed to speed the absorption of insulin into the bloodstream and reduce A1C levels, was approved by the FDA in June 2020.

XTalks
NOVEMBER 3, 2022
Demand for Lilly’s GIP/GLP-1 receptor agonist Mounjaro is also rising because of high patient demand since the drug’s May 13 FDA approval and expanding insurance coverage. Mounjaro has thus taken off stronger than expected, surpassing Wall Street sales forecasts during the third quarter.

XTalks
FEBRUARY 21, 2024
There are currently no FDA-approved drug therapies for MASH, and the disease is characterized by excess fat accumulation and inflammation in the liver, which leads to liver scarring or fibrosis. It has been approved for treating diabetes and obesity.

pharmaphorum
NOVEMBER 21, 2022
The company argues that the cost is justified as Tzield (teplizumab) is the first drug that can delay the onset of type 1 diabetes, fending off the time when they become highly reliant on insulins and at risk of the serious complications that can accompany advanced T1D.

Pharmaceutical Technology
DECEMBER 22, 2022
Sanofi wins the Product Launches award this year following the further development of its Dupixent (dupilumab) product and a string of approvals. In May the drug became the first medicine available in the US for eosinophilic oesophagitis following approval. Civica JAAQ Sanofi. The post Excellence Awards 2022 – Winners Announced!
STAT News
DECEMBER 5, 2022
Meantime, the FDA told ImmunoGen and ADC Therapeutics, which have been developing cancer drugs, that it would not grant a speedy approval until they had begun follow-up studies. Advocates and legal experts say the suit has no merit, but they fear conservative courts will think otherwise.

Drug Discovery World
NOVEMBER 16, 2022
Secreted proteins are a proven class of biologics with largely underutilised therapeutic potential, despite the impact of FDA-approved biologics based on secreted proteins such as insulin, human growth hormone, and erythropoietin.

XTalks
JULY 3, 2023
The treatment is derived from deceased donor pancreatic cells and is indicated for patients who are unable to achieve average blood glucose levels (glycated hemoglobin) with daily insulin injections or with continuous infusion through a pump because of repeated episodes of severe hypoglycemia (low blood sugar).
pharmaphorum
FEBRUARY 21, 2022
Since the FDA approval, Bayer has also reported the results of a second phase 3 trial – FIGARO-DKD – that concentrated primarily on cardiovascular endpoints and also includes patients with earlier-stage kidney disease.

pharmaphorum
APRIL 1, 2022
And already in the first quarter of 2022, the FDA approved a third filgrastim biosimilar, Releuko. With 34 approved biosimilars and dozens more in the pipeline, what does this year have in store? In September of 2021, the FDA approved Byooviz , which references Roche and Novartis’ blockbuster eye drug Lucentis.

pharmaphorum
FEBRUARY 18, 2021
The history of insulin captures one of the mystifying complexities of the pharmaceutical market — how long-standing drugs become more expensive with time and competition fails to hold down prices. 6 The stakes were extremely high, with annual costs of insulin reaching $736 per patient in 2013, up threefold since 2002.
XTalks
OCTOBER 11, 2023
They mimic the action of GLP-1, a hormone that helps regulate blood sugar levels by enhancing insulin secretion. Ozempic and Wegovy continue to be blockbusters for Novo, despite facing competition from Eli Lilly’s dual GLP-1/GIP agonist Mounjaro (tirzepatide), which won FDA approval in May 2022.

Drug Discovery World
JULY 5, 2023
Lantidra provides a potential treatment option for patients with type 1 diabetes who have trouble managing the amount of insulin needed every day to prevent hyperglycaemia (high blood sugar) without causing hypoglycaemia. Five participants did not achieve any days of insulin independence.
pharmaphorum
MARCH 3, 2022
Non-profit drugmaker Civica Rx has said it will launch biosimilars of three big-selling insulin products in the US by 2024 to help diabetic patients struggling with the cost of the drugs. Cheaper options are meanwhile becoming available.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content