Novel method for producing genetically modified measles viruses for vaccine research
Medical Xpress
NOVEMBER 29, 2022
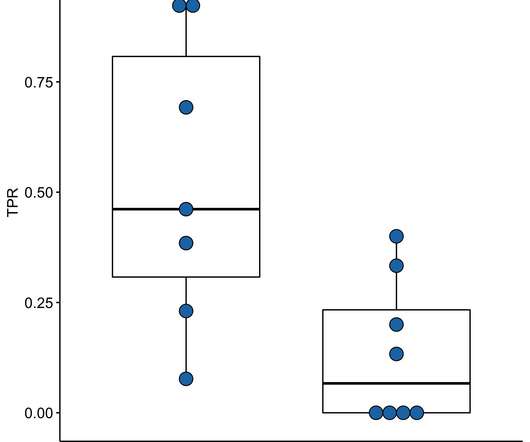
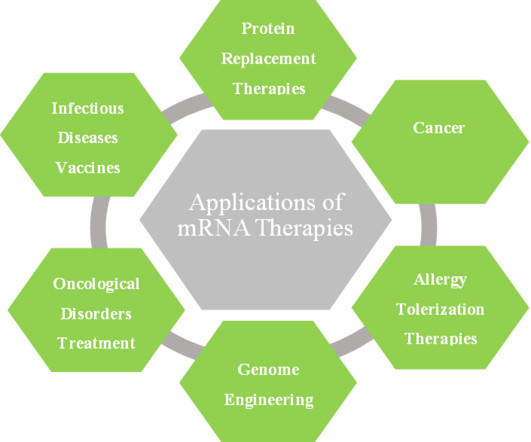
Genetically modified and attenuated measles viruses are considered a promising platform for research and development of vector vaccines and oncolytic measles viruses.






























Let's personalize your content