Promising results from in vitro combination therapy against COVID-19
Scienmag
NOVEMBER 17, 2020
Researchers at Karolinska Institutet in Sweden report promising results from an in vitro combination therapy against COVID-19.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
MAY 4, 2023
The approval allows KALYDECO to be used in infants who have at least one mutation in their cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to the therapy, on the basis of clinical and/or in vitro assay results. The medicine is currently available for use in more than 30 countries.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
SEPTEMBER 19, 2022
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorisation for AstraZeneca ’s Evusheld (formerly AZD7442) for Covid-19 in the European Union (EU). Additionally, in the trial, the antibody cocktail was found to be well-tolerated.
The Pharma Data
OCTOBER 16, 2020
As pharma companies search for solutions to avoid cancelling or delaying clinical trials, virtualizing trials are fast becoming commonplace during the Covid-19 pandemic. With many nationwide and regional lockdowns coming into force, virtual clinical trials are proving to be an effective way to monitor patients remotely.

Drug Discovery World
MARCH 8, 2024
Biopharmaceutical company Innovent Biologics has launched the first-in-human (FIH) Phase I clinical trial of IBI3002, a novel bispecific antibody targeting Interleukin 4 receptor α (IL-4Rα) and thymic stromal lymphopoietin (TSLP). In vitro assays have shown superiority over the marketed monoclonal antibodies to respective target.
Drug Discovery World
JULY 31, 2023
DDW’s Reece Armstrong speaks to Hendrik Streefkerk , Chief Medical Officer and Drug Safety Officer of CellProthera about the company’s cell therapy for acute myocardial infarction (AMI) and why regenerative medicine could be key to treating cardiac diseases. Unfortunately, this is not enough,” Dr Streefkerk states.

Pharmaceutical Technology
APRIL 12, 2023
Foundation Medicine has announced it will supply a tissue-based test as a companion diagnostic for Bristol Myers Squibb’s (BMS) recently acquired ROS1/TRK inhibitor repotrectinib. FoundationOne CDx is a sequencing-based in vitro diagnostic device that can identify alterations in 324 genes from tumour samples.

The Pharma Data
MARCH 3, 2022
Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced an initial donation of essential medicines to Ukraine. At the same time we are ensuring that our critical medicines and diagnostics reach the people who need them both in Ukraine and other countries impacted by the crisis. Roche vehemently condemns the violent invasion of the country.

pharmaphorum
JANUARY 25, 2022
As the area of bringing a digital therapeutic to market means passing the same level of regulatory scrutiny as a traditional, pharmaceutical therapy, this means “prescription digital therapeutics must undergo extensive clinical trials to demonstrate their safety and effectiveness before they ever reach patients,” said McCann.

Pharmaceutical Technology
MAY 31, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It provides medicines for the treatment of cancer, other auto-immune diseases, central nervous system disorders, ophthalmological disorders, infectious diseases, and respiratory diseases.

XTalks
NOVEMBER 7, 2022
Gleich, MD, FACS, Senior Vice President, Medical Department, and Dr. Christopher Huth, PhD, Senior Clinical Trial Manager, Clinical Trial Management. Liquid Biopsy Use in Oncology Clinical Trials. These tumor-derived entities are used to derive genomic and proteomic data. Concentration of CTCs in Cancer Patients.
Drug Discovery World
APRIL 25, 2023
University of California researchers have dosed the second participant in their clinical trial to identify a potential cure for HIV utilising CAR-T cell therapy. The trial is the first-in-human clinical study investigating the duo CAR-T cell therapy for the treatment of HIV. In 2021 there were an estimated 1.5

Drug Discovery World
APRIL 8, 2024
Outstanding Achievement Award Pfizer Chairman and CEO Dr Albert Bourla received the 2024 AACR Outstanding Achievement Award for Service to Cancer Science and Medicine on behalf of Pfizer during the Opening Ceremony.

The Pharma Data
AUGUST 24, 2020
Parties to adopt multi-phase approach to develop precision medicine strategies to optimize treatment of cardiovascular, renal and metabolic disease. AstraZeneca has linked with RenalytixAI to develop and launch precision medicine strategies for cardiovascular, renal and metabolic diseases. Source link.
Drug Discovery World
JUNE 30, 2023
Research tools, safety testing and regenerative medicines – these endless stem cell applications are powering precision, speed, and new modalities in drug development. Stem cells for use in drug discovery Stem cells are fast becoming an invaluable tool in the drug discovery process. This article is sponsored by Charles River.

XTalks
APRIL 2, 2021
Pfizer released new COVID-19 vaccine trial results this week from its ongoing clinical studies, which include data showing that its COVID-19 mRNA vaccine is 100 percent effective in children between the ages of 12 and 15 and has a 91 percent efficacy against variants with protection lasting at least six months.

Pharmaceutical Technology
JUNE 5, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It provides medicines for the treatment of cancer, other auto-immune diseases, central nervous system disorders, ophthalmological disorders, infectious diseases, and respiratory diseases.
Drug Discovery World
OCTOBER 25, 2023
Data generated by our laboratory across in vitro and in vivo models and from human clinical samples support this important finding. Previous attempts to interrupt TGF-β pathway signaling in cancer have been thwarted by activation of alternative resistance pathways by the tumour.

pharmaphorum
FEBRUARY 13, 2022
Quris’ BioAI safety prediction platform – which is based on human tissue samples on chips, nanosensors and machine learning – will be compared to traditional in vitro and in vivo laboratory techniques for spotting toxicity. The post Merck will assess Quris’ AI ‘patient-on-a-chip’ drug safety appeared first on.
Roots Analysis
AUGUST 18, 2023
Due to this biological mismatch, several toxic drugs are subjected to costly clinical trials, while potentially useful drugs do not get commercialized. In order to fasten the discovery of innovative drugs and personalized medication, improved in vitro simulation of human biology and pathologies is required.

Drug Discovery World
APRIL 5, 2023
A collaborative network The CBC operates as a collaborative network of 19 Institutes with longstanding drug discovery know-how from target identification through to proof-of-concept clinical trials. The post Only non-US company joins cancer drug discovery consortium appeared first on Drug Discovery World (DDW).
pharmaphorum
MARCH 4, 2021
Sunak pledged £28 million towards the UK’s capacity for vaccine testing, support for clinical trials and to improve the ability to acquire samples of new COVID-19 variants. ” BIVDA, the trade association for British in-vitro diagnostic companies, supported the “super-deduction” policy.

XTalks
AUGUST 9, 2023
This new medicine is a positive step forward for the treatment of this disease in many patients who have been struggling for years,” said Christopher Starr, MD, associate professor of ophthalmology at Weill Cornell Medicine, New York Presbyterian Hospital, in the company’s press release. California-based Tarsus Pharmaceuticals Inc.,
XTalks
JANUARY 9, 2023
Its mechanism of action differs from currently approved antivirals and has thus far shown no cross-resistance in vitro to other drug classes. Sunlenca received New Drug Application (NDA) approval based on the results of the CAPELLA trial, in which it demonstrated high rates of sustained virological suppression. Gilead Sciences, Inc.

Drug Discovery World
AUGUST 22, 2023
Claire D’Abreau-Hayling, Chief Scientific Officer at Sandoz, examines how generic products can relieve industry pressure and improve access to essential medicines. Off-patent medicines today account for about 80% of global prescriptions at an estimated 20% of the total cost. What are complex generics?

The Pharma Data
MARCH 25, 2022
This includes about three million people in the EU who are immunocompromised or being treated with immunosuppressive medicines.(1). 2-4 Evusheld was generally well-tolerated in the trial.(2-4). 2-4 Evusheld was generally well-tolerated in the trial.(2-4). 2 Omicron SARS-CoV-2 subvariants in circulation around the world.(5-7)

Drug Discovery World
SEPTEMBER 28, 2023
Earlier this year, we explored the microbiome and its role in drug development across various therapeutic areas. This article will focus on one such area of great unmet need and promise: women’s health. This is likely in part because their byproduct, lactic acid, is known to inhibit pathogenic bacteria 25.
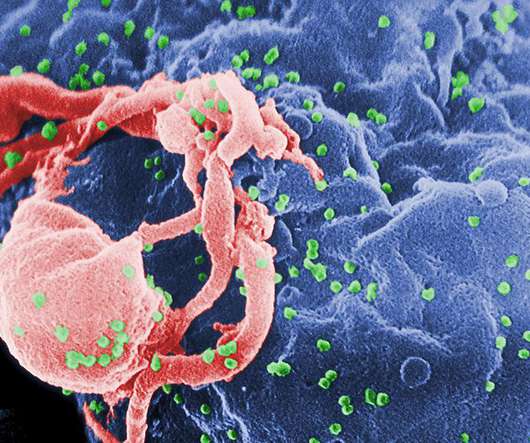
pharmaphorum
FEBRUARY 19, 2021
Excision wants to take its lead therapy candidate, EBT-101, into a phase 1/2 clinical trial in patients with chronic HIV infection with the proceeds from this latest financing round. We have proven the technology and candidate programs in vitro and in vivo in both small animal models and primate models.

XTalks
MARCH 1, 2021
NYSE: PFE ) and BioNTech SE (Nasdaq: BNTX ) have announced that they are beginning a trial to evaluate the safety and efficacy of a third booster dose for their COVID-19 vaccine (BNT162b2), as well as new vaccine variants. The study will be a part of current Phase I, II and III trials. Pfizer Inc.

Drug Discovery World
JANUARY 23, 2023
This action sensitised GBM cells to radiotherapy both in vitro and in vivo (in mouse models). “Our research has revealed cladribine as a radiosensitiser for GBM treatment by drug repurposing, which can offer multiple advantages,” says Prof Youn. “As GBM is a WHO grade IV brain tumour with dismal prognosis.

The Pharma Data
OCTOBER 14, 2021
This designation is based on data showing that gantenerumab significantly reduced brain amyloid plaque, a pathological hallmark of AD, in the ongoing SCarlet RoAD and Marguerite RoAD open-label extension trials, as well as other studies. Roche’s Chief Medical Officer and Head of Global Product Development.

The Pharma Data
JANUARY 19, 2021
TC-210 is currently in a Phase I/II trial for mesothelin-positive non-small cell lung cancer (NSCLC), ovarian cancer, malignant pleural/peritoneal mesothelioma, and cholangiocarcinoma. TC-110 is in a Phase I/II trial for CD19-positive adult acute lymphoblastic leukemia (aALL) and with aggressive or indolent non-Hodgkin lymphoma (NHL).

The Pharma Data
MARCH 29, 2022
Evusheld significantly reduced the risk of developing symptomatic COVID-19 in PROVENT Phase III trial, with protection lasting at least six months Evusheld retains neutralising activity against the Omicron BA.2 1-3 Evusheld was generally well-tolerated in the trial. 1-3 Evusheld was generally well-tolerated in the trial.
pharmaphorum
NOVEMBER 9, 2022
for the purpose of advancing disease-modifying oral medicines for debilitating chronic neurodegenerative disorders, by enabling investigational new drugs (IND) studies. A £16 million Series A financing – led by Omega Funds – has been announced by the innovative neuroscience company NRG Therapeutics Ltd.,
Drug Discovery World
MAY 9, 2023
Fewer than 5% of oncology drugs that enter clinical trials in the US receive US FDA approval. This abysmal success rate significantly trails the overall approval rate for compounds entering human trials (about 10% of which reach commercialisation). Cancer is far more complex than the sum of its parts.

pharmaphorum
SEPTEMBER 9, 2020
The Wavre-based biotech says it has conducted clinical trials in around 2,000 patients to see how effective Sedakit is at relieving pain and anxiety without the use of drugs. She came up with the idea for Sedakit after living through the stress of watching one of her own family members fight cancer.

The Pharma Data
OCTOBER 9, 2021
This designation is predicated on data showing that gantenerumab significantly reduced brain amyloid pillar, a pathological hallmark of Notice, in the ongoing SCarlet RoAD and Marguerite RoAD open- tag extension trials, as well as other studies. Roche’s Chief Medical Officer and Head of Global Product Development.

The Pharma Data
APRIL 4, 2022
More than one million people with severe or critical COVID-19 have already been treated with Actemra/RoActemra worldwide, demonstrating the important role of this medicine in the fight against the pandemic.”. A decision on U.S. FDA approval is expected in the second half of this year. Actemra/RoActemra is not U.S.

The Pharma Data
MAY 17, 2023
Additionally, pre-clinical data have shown fenebrutinib to be potent and highly selective, and it is the only reversible inhibitor currently in Phase III trials for MS. “I Fenebrutinib significantly reduced the total number of new gadolinium-enhancing T1 brain lesions compared to placebo, the primary endpoint of the trial (p=0.0022).

The Pharma Data
SEPTEMBER 1, 2020
Antigen test reliably and quickly triages people suspected of SARS-CoV-2, with results ready in 15 minutes, allowing informed treatment decisions. Antigen test accurately screens individuals with known exposure to infected SARS-CoV-2 patients, providing fast answers regarding their infection status. Food and Drug Administration (FDA).

The Pharma Data
AUGUST 18, 2020
REGN-COV2 is Regeneron’s two-antibody combination currently in late-stage clinical trials for the treatment and prevention of COVID-19 infection. If it proves safe and effective in clinical trials and regulatory approvals are granted, Regeneron will distribute and record sales for REGN-COV2 in the U.S. and around the world.

The Pharma Data
SEPTEMBER 17, 2020
The new quantitative Elecsys antibody test can play a pivotal role in vaccine clinical trials as well as helping clinicians assess patients immune response.This will be instrumental in protecting people most vulnerable to the virus, as well as in overcoming COVID-19 for society in general.” Food and Drug Administration (FDA).

The Pharma Data
MAY 27, 2022
Data from the NP30179 study have been submitted for approval to the European Medicines Agency (EMA), and submissions to additional health authorities worldwide, including to the U.S. Glofitamab is being investigated in several clinical trials and explored in earlier lines of lymphoma treatment. After a median follow-up of 12.6

The Pharma Data
MAY 31, 2022
More than 5,000 patients have now been treated worldwide with Evrysdi in clinical trials, compassionate use or real-world settings. Evrysdi is now approved in the US to treat SMA in children and adults of all ages. RAINBOWFISH Principal Investigator and Director of the Experimental Neuroscience Program at St.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content