Arecor hopes London stock market float will raise profile of insulin products
pharmaphorum
MAY 17, 2021
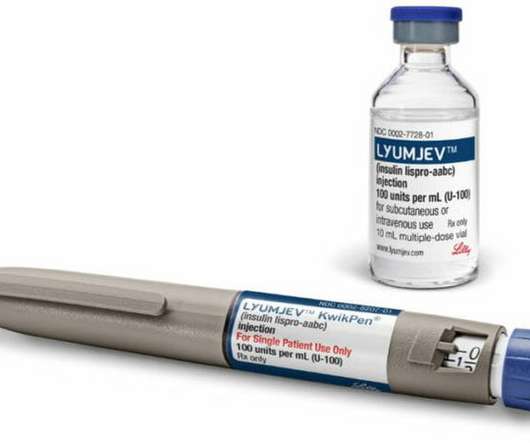
UK biotech Arecor Therapeutics has announced plans to float on the London stock market, to fund plans to develop novel formulations of insulins and other biological drugs with enhanced properties. Arecor has four active licensing agreement in place with partners including Hikma and Inhibrx.









































Let's personalize your content