Packaging Solutions for Pharmaceuticals & Medical Devices: Insights and Considerations
XTalks
MAY 13, 2022
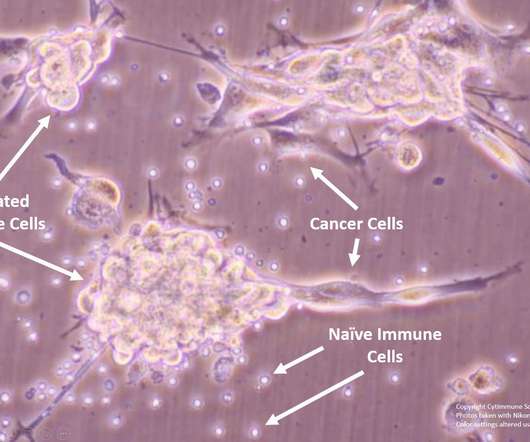
A container closure system consists of all the packaging components that contain and protect a pharmaceutical product. Container closure systems are highly regulated by health agencies. They provide specific guidance on packaging requirements for closed closure integrity (CCI) and general integrity testing.




































Let's personalize your content